Chapter Eleven
Women and Congestive Heart Failure
Congestive heart failure (CHF) is a severe consequence of cardiovascular disease. In general CHF can be caused by coronary artery disease (CAD) and myocardial infarction (MI) as well as a plethora of other non-ischemic factors. CHF is the number one cause of hospitalization in the US today and accounts for billions of dollars in healthcare expenditures each year. In the setting of CAD and post-MI, the lifetime risk for the development of CHF is identical in men and women and is roughly one in five. However, in non-MI CHF, the lifetime risk is much higher in women than in men — one in six as compared to one in nine.1 Most experts agree that other causes of CHF, such as long-standing, untreated hypertension, may be to blame for the high rate of non-MI-related CHF in women. Many women do not adequately address basic health issues such as treatment of hypertension and this may explain the disproportionately high rates of CHF in women. There have been multiple epidemiological studies that have suggested that there may be gender differences in progression and prognosis in CHF once it develops.2 The reasons for these gender differences in prognosis are not clear — it may be that women present later with more severe left ventricular dysfunction and more advanced CHF.
Data has shown that women tend to present later with CHF and often have more diastolic dysfunction than men. In addition, female patients with CHF have greater functional impairment, more hospi-talizations, overall lower quality of life and increased rates of depression when compared to men with similar disease burdens.3, 4 Women are much more likely to have CHF with preserved left ventricular function but do seem to have overall lower mortality rates.
To put these statistics in perspective, a woman’s lifetime risk of developing breast cancer is one in nine — much lower than the lifetime risk of CHF.5 As mentioned previously in the book, women tend to believe that their greatest health risk comes from breast and uterine cancer when, in reality, their greatest risk is from heart disease and its complications. With improved treatments for heart disease and other medical advancements that are promoting longevity in the population, it is expected that the rates of CHF will continue to grow at an alarming rate. Strategies have been developed to work at primary prevention of CHF related to hypertension and seem to be effective — some studies even tout a 50% reduction in incidence of CHF in populations where hypertension is aggressively managed.6 We now have well-proven therapies for the treatment of acute MI. In addition, drugs such as ACE inhibitors and beta-blockers — when used properly in the post-MI period — will promote remodeling of the heart muscle and ultimately reduce the incidence of CHF.
Common causes of CHF
Congestive heart failure has many identified causes. CHF can either be due to systolic dysfunction or related to diastolic or relaxation abnormalities. Both varieties are associated with significant morbidity and mortality as well as extensive costs to the healthcare system. In systolic dysfunction, the left ventricular ejection fraction is reduced and the ventricle is no longer able to meet the demands of contraction and perfusion — resulting in congestion, edema and clinical symptoms consistent with CHF. In diastolic dysfunction, certain insults result in the impairment of ventricular relaxation during diastole and subsequent congestion and similar clinical symptoms. The more common etiologies of CHF are varied but include:
1. Coronary artery disease/Ischemic heart disease
2. Hypertension
3. Valvular heart disease
4. Idiopathic dilated cardiomyopathy
5. Congenital heart disease
6. Infiltrative disease (sarcoidosis, amyloidosis)
7. Others (anemia, thyroid disease, etc)
While the most common etiology appears to be CAD, each of these disorders can produce CHF to some degree — all are associated with significant morbidity and mortality.
Coronary artery disease: CAD and post-MI CHF is quite common. In the setting of CAD and MI, the patient’s left ventricular ejection fraction is reduced due to infarction and necrosis of healthy cardiac myocytes during an acute cardiovascular event. During the post-MI period, much of the formerly healthy heart muscle is replaced with scar and fibrous tissue. These areas of infarction, if extensive, can impair the overall contractility of the heart. In some circumstances, such as when the area of the heart containing the left bundle branch is involved, the contractility of the heart can become dyssynchronous — further impairing the efficiency of the heart during systole.
Hypertension: Hypertension is one of the more common causes of CHF.7 In the setting of long-standing hypertension, the heart makes adaptive changes over time. According to Starling’s law of the heart, there comes a point at which the muscle cells can no longer continue to compensate for the increased wall stress and pressure that is imparted by elevated arterial pressures. The heart can become dilated and the ejection fraction can fall — systolic CHF then ensues. Alternatively, the heart can become stiff and relaxation is limited, resulting in diastolic heart failure. Prognosis following the onset of hypertension-related CHF is poor — according to a study in JAMA, only 24% of men and 31% of women were alive five years after diagnosis.8
Valvular heart disease: Valvular heart disease can have different effects on the heart and can result in clinical CHF. Depending on the particular type of valvular abnormality, symptoms can range from mild to severe CHF and can develop slowly over time or present acutely and in dramatic fashion. Most CHF associated with vavlular heart disease is systolic in nature, although there are situations that may produce relaxation abnormalities and result in diastolic CHF. The most common valvular lesions associated with the development of CHF are aortic stenosis and mitral regurgitation and tend to occur more frequently in patients over the age of 65.9 Interestingly, a study from The Lancet in 2006 indicates that even though prevalence of disease is similar and not gender specific, women are less likely to be diagnosed with and treated for valvular heart disease.10
Idiopathic dilated cardiomyopathy (IDCM): IDCM is common in the world today. In nearly 50% of cases of CHF, the etiology remains unknown and these patients are often termed idiopathic cardiomyopathies.11 IDCM may be caused by numerous agents, including infection, toxic insults, genetic/inherited conditions, as well as inflammatory mediated events. In women, an important clinical syndrome associated with IDCM is the peri-partum cardiomyopthy (PPCM). In PPCM, women develop symptoms of CHF within the last month of pregnancy or within five months of delivery and left ventricular dysfunction is often severe. These pregnancy-related cardiomyopathies have a variable course — some resolve with delivery whereas others begin and persist in the post-partum period.12 PPCM occurs in women with no pre-existing cardiovascular disease.
Congenital heart disease (CHD): As therapies for CHD continue to improve, many patients are living well into adulthood with significant congenital lesions. Over time, some of these patients do in fact develop left ventricular dysfunction and CHF related to their particular CHD lesion. In addition, women with CHD must be carefully managed during pregnancy as pregnancy-induced changes in the cardiovascular system can have particularly severe implications for women. In addition, women with CHD who are pregnant are at increased risk for offspring with CHD as compared to the general population.
Infiltrative diseases: Infiltrative diseases such as sarcoidosis and amyloidosis can produce restrictive cardiomyopathies. Patients with infiltrative diseases can have both diastolic as well as systolic dysfunction. These patients are at increased risk for ventricular arrhythmias and many present with syncope or sudden death syndromes at initial diagnosis.13 Sarcoidosis occurs most commonly between the ages of 20 and 30 and is much more common in women than in men. Amyloidosis is a disease in which certain proteins are deposited in organs and replace normal healthy tissues with amyloid protein. In the case of cardiac amyloidosis, patients experience thickening of the left ventricle and often present with signs and symptoms consistent with CHF.
Other causes of CHF: Other diseases can contribute to the development of CHF. Some of these causes are quite reversible and may be related to metabolic abnormalities such as thyroid disease. Thyroid disease and related CHF is more common in women than in men. Other causes include severe anemia and viral infections (such as coxackievirus). Rare contributing factors include abnormalities of iron metabolism such as hemochromatosis, as well as inflammatory processes that result in a global myocarditis. While the above is not exhaustive, it does address most of the known causes of CHF.
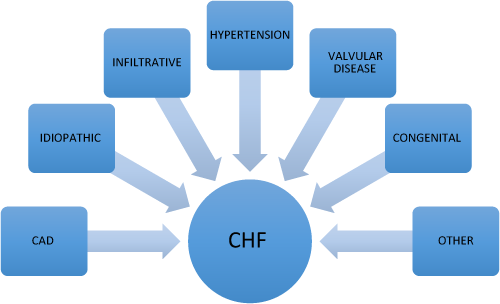
Figure 11.1 Common causes of CHF.
Common symptoms associated with CHF
CHF can manifest in many different ways. Most commonly CHF symptoms are related to congestion and include shortness of breath, dyspnea on exertion and edema. Although the symptoms of CHF are similar in men and women, females tend to have more shortness of breath and peripheral edema as compared to their male counterparts. In addition, women also have more difficulty with exertional dyspnea as compared to men.
The cardinal signs and symptoms of CHF include:
1. Shortness of breath
2. Fatigue
3. Dyspnea on exertion
4. Peripheral edema
5. Positional nocturnal dyspnea and orthopnea
6. Cough
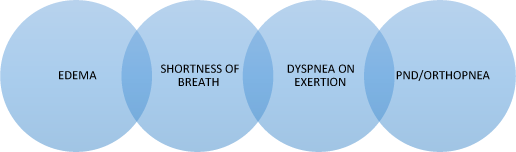
Figure 11.2 Common symptoms of CHF. PND = postural nocturnal dyspnea.
Symptoms may be slowly progressive or may occur very quickly, depending on the etiology of CHF.
Gender differences in risk factor effects
Not all risk factors have equivalent effects in both men and women. As clinicians and as patients, we must consider risk factors in the context of their variable gender-specific effects on lifetime risk for CHF. It is important to understand the impact of different risk factors on women and prioritize interventions in order to make the biggest impact on risk reduction in female patients.
Common risk factors for CHF include CAD, hypertension, diabetes, obesity, elevated cholesterol and smoking.14 These risk factors seem to have important gender-specific effects. For example, hypertension confers much greater risk for CHF in women than in men. Although the exact mechanism by which hypertension affects women differently is not known, it may be that the afterload increase that occurs with hypertension has a greater effect on women’s hearts and results in more ventricular dysfunction — predisposing females to more CHF.15 Interestingly, CAD is more prevalent in men with CHF as compared to women. Myocardial infarction is a much more common risk factor for CHF in men — however, in women who do have CAD and suffer a MI, CHF is much more common.16 In addition, women who have had an MI and subsequently have been revascularized with coronary artery bypass grafting (CABG) are much more likely to develop CHF than men.17 The presence of diabetes appears to have a very powerful effect for the development of CHF in women. Numerous studies have shown a direct correlation between the presence of diabetes and the development of CHF. When the rates of concomitant diabetes in women with CHF are compared to similar male patients, women had an eight-fold increase in incident heart failure and men only a four-fold increase. Although no mechanism for this effect is known, based on Framingham data, it is postulated that diabetes may produce increased wall thickness and left ventricular mass specific to women — thus contributing to diastolic relaxation abnormalities and clinical CHF in women.18 In general, smoking appears to have a greater effect on men than in women — in fact, smoking appears to be less common in female CHF patients than in men — although some studies have shown that smoking seems to increase CHF risk in young men and older women with CHF.19, 20 Obesity has been found to confer increased risk for CHF in both sexes, however, data from Framingham suggests that women who are obese may suffer even greater risk.10,11 Dyslipidemia has also been associated with a higher risk for CHF in women as compared to men.
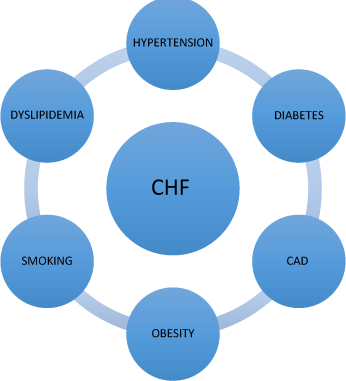
Figure 11.3 Risk factors for CHF.
Sex differences in response to therapy for CHF
Historically, most CHF trials have included a minority of women as subjects. Much of the data that we use today to recommend therapy are not gender specific due to under-representation of women in the clinical trials. However, the best available data suggests that we should treat men and women in a similar fashion (until such time as gender-specific trials in CHF are conducted and published). Several clinical investigations have noted a difference in the way in which men and women respond to particular therapies. For example, men tend to gain a larger survival benefit from ACE inhibitors as compared to women.21 In addition, meta-analysis of ACE inhibitor trials does indicate that women with CHF who are symptomatic derive more benefit from ACE inhibitors than those who were asymptomatic.22 Certain angiotensin receptor blockers (ARBs) such as candesartan and valsartan appear to be beneficial in women and have been shown to reduce cardiovascular death and hospitalization in women.23 Some studies indicate that women have greater responses from beta-blockers due to sex-related differences in the way in which the drugs act in vivo — differences in pharmacokinetics may result in greater drug exposure in women.24 The combination of beta-blockers and ACE inhibitors seems to be particularly effective in female patients with CHF. In this case, even though women and men may take the same dose of beta-blockers, women seem to achieve a greater benefit and ultimately may have better responses to beta-blockers in CHF.
Special circumstances: depression and CHF
Depression has been associated with CHF and the effective treatment of depression in CHF patients has had a significant impact on hospitalization and outcomes. The overall prevalence of depression in CHF ranges from 24% to 42%.25 Numerous studies have also shown that the prevalence in depression among patients hospitalized with CHF is significantly higher in women.26 More concerning is the fact that CHF patients with major depression are than twice as likely to die or be readmitted to the hospital as compared to those without depression at all.27 Clearly, depression represents a major determinant of outcome in CHF — however, many patients are not diagnosed or adequately treated. As healthcare providers who treat patients with CHF, we must become more vigilant and work to ensure that we identify patients at risk for depression and provide them with proper therapies for depression once it is identified.
1 Lloyd-Jones, D. M. (2002). Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation, Volume 106, 3068–3072.
2 Ho, K. K., Anderson, K. M., Kannel, W. B. et al. (1993). Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation, Volume 88, 107–115.
3 Deswal, A. and Bozkurt, B. (2006). Comparison of morbidity in women versus men with heart failure and preserved ejection fraction. Am J Cardiol, Volume 97, 1228–1231.
4 Gottlieb, S. S., Khatta, M., Friedmann, E. et al. (2004). The influence of age, gender, and race on the prevalence of depression in heart failure patients. J Am Coll Cardiol, Volume 43, 1542–1549.
5 Feuer, E. J., Wung, L., Boring, C. C. et al. (1993). The lifetime risk of developing breast cancer. J Natl Cancer Inst, Volume 85, 892–897.
6 Senni, M., Triboiuilloy, C. M., Rodeheffer, R. J. et al. (1999). Congestive heart failure in the community: Trends in incidence and survival in a 10-year period. Arch Intern Med, Volume 159, 29–34.
7 Levy, D., Larson M. G., Vasan, R. S. et al. (1996). The progression from hypertension to congestive heart failure. JAMA, Volume 275, 1557–1562.
8 Ibid.
9 Rich, M. W. (1997). Epidemiology, pathophysiology, and etiology of congestive heart failure in older adults. Journal of the American Geriatrics Society, Volume 45(8), 968–974.
10 Nkomo, V. T. (2006). Burden of valvular heart diseases: A population-based study. The Lancet, Volume 368(9540), 1005–1011.
11 Hazebroek, M. (2012). Idiopathic dilated cardiomyopathy: Possible triggers and treatment strategies. Neth Heart J, 20(7–8), 332–335.
12 Pearson, G. D., Veille, J. C., Rahimtoola, S. et al. (2000). Peripartum cardiomyopathy: National Heart, Lung, and Blood Institute and Office of Rare Diseases (National Institutes of Health) workshop recommendations and review. JAMA, Volume 283, 1183–1188.
13 Reuhl, J., Schneider, M., Sievert, H. et al. (1997). Myocardial sarcoidosis as a rare cause of sudden cardiac death. Forensic Sci Int, Volume 89(3), 145–153.
14 Petrie, M. C. (1999). Failure of women’s hearts. Circulation, Volume 99, 2334–2341.
15 Garavaglia, G. E., Messerli, F. H., Schmieder, R. E. et al. (1989). Sex differences in cardiac adaptation to essential hypertension. Eur Heart J, Volume 10, 1110–1114.
16 Tofler, G. H., Stone, P. H., Mueller, J. E. et al. (1987). Effects of gender and race on prognosis after myocardial infarction: Adverse prognosis for women, particularly black women. J Am Coll Cardiol, Volume 9, 473–482.
17 Hoffman, R. M., Psaty, B. M. and Kronmal, R.A. (1994). Modifiable risk factors for incident heart failure in the Coronary Artery Surgery Study. Arch Intern Med, Volume 154, 417–423.
18 Galderisi, M., Andersson, K. M., Wilson, P. W. F. et al. (1991). Echocardiographic evidence for the existence of a distinct diabetic cardiomyopathy: The Framingham Heart Study. Am J Cardiol, Volume 68, 85–89.
19 Kannel, W. B. (1989). Epidemiological aspects of heart failure. Cardiol Clin, Volume 7, 1–9. 20 Kannel, W. B. and Belanger, A. J. (1991). Epidemiology of heart failure. Am Heart J, Volume 121, 951–957.
21 Petrie, M. C., Dawson, N. F., Murdoch, D. R. et al. (1999). Failure of women’s hearts. Circulation, Volume 99, 2334–2341.
22 Shekelle, P. G., Rich, M. W., Morton, S. C. et al. (2003). Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol, Volume 41, 1529–1538.
23 Young, J. B., Dunlap, M. E., Pfeffer, M. A. et al. (2004). Mortality and morbidity reduction with Candesartan in patients with chronic heart failure and left ventricular systolic dysfunction: Results of the CHARM low-left ventricular ejection fraction trials. Circulation, Volume 110, 2618–2626.
24 Luzier, A. B., Killian, A., Wilton, J. H. et al. (1999). Gender-related effects on meto-prolol pharmacokinetics and pharmacodynamics in healthy volunteers. Clin Pharmacol Ther, Volume 66, 594–601.
25 Havranek, E. P., Ware, M. G. and Lowes, B. D. (1999). Prevalence of depression in congestive heart failure. Am J Cardiol, Volume 84, 348–350.
26 Freedland, K. E. (2003). Prevalence of depression in hospitalized patients with congestive heart failure. Psychosomatic Medicine, Volume 65(1), 119–128.
27 Jiang, W., Alexander, J., Christopher, E. et al. (2001). Relationship of depression to increased risk of mortality and rehospitalization in patients with congestive heart failure. Arch Intern Med, Volume 161(15), 1849–1856.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


