Condition
Points
C
CHF
1
H
HTN
1
A2
Age ≥75 years
2
D
Diabetes mellitus
1
S2
Prior stroke or TIA or TE
2
V
Vascular disease
1
A
Age 65–74 years
1
SC
Sex (female gender)
1
The role of the competing strategies of rate control versus rhythm control remains an unsettled issue. In the perfect world, most practitioners would opt for rhythm control. The publication of the AFFIRM [8] and RACE [9] trials in 2002 demonstrated that rate control was not inferior to rhythm control with medication and that rate control may be advantageous to rhythm control. As a result, rate control became the preferred strategy in the majority of AF cases. With the advent of catheter ablation of AF the pendulum appears to be shifting back toward rhythm control. However, due to a lack of a well-designed large randomized study related to ablation with long term follow-up and hard clinical end points of morbidity and mortality to establish its clinical benefit, its benefit for rhythm control remains unsettled.
Mechanisms of Atrial Fibrillation
There are two main mechanisms involved in the genesis and maintenance of AF: 1-the multiple wavelet hypothesis with large and small reentrant wavelets, 2- the focal trigger hypothesis with enhanced automaticity of 1 or several rapidly depolarizing foci [5]. In most cases, it is the combination that results in the development of AF with focal triggers leading to the initiation of reentry that eventually leads to atrial remodeling causing additional focal triggers and perpetuation of reentry.
Until the 1980’s, the multiple wavelet hypothesis was widely accepted as the dominant mechanism. The development of the surgical Cox-Maze procedure, first performed in 1987, was predicated on this AF model and the concept that maintenance of AF needs a critical number of circulating wavelets, each of which requires a critical mass of atrial tissue [5]. The multiple thru and thru surgical atrial incisions were designed to interrupt all macro-reentry circuits preventing the ability of the atrium to fibrillate. Procedural success contributed to development of catheter ablation with creation of multiple ablation lines to interrupt reentry.
The focal trigger hypothesis became a major factor for the initiation of AF in the 1990’s with identification of rapidly depolarizing ectopic foci in the atrium and pulmonary veins that would trigger AF or act as a rapid driver to maintain AF. These ectopic foci usually originated from myocardial muscle tissue found in the proximal 1–3 cm of the pulmonary (PVs) veins [5] and became the target of catheter ablation.
History and Rationale of AF Catheter Ablation
In 1994, Michel Haissaguerre presented the first successful catheter ablations of AF in three patients [10]. He found rapidly firing foci in the right atrium and successfully treated them with RF catheter ablation. These results supported the concept of a focal mechanism for AF that can be treated by ablation.
The concept of focal triggers was further supported in 1998 in another study by Haissaguerre et al. [11]. They found that the vast majority of the focal triggers of AF were in the proximal portion of the PVs. In the 45 patients studied, a total of 69 triggering foci were found of which 64 (94 %) were found in the proximal few centimeter of the PVs: 31 foci in the left superior, 17 in the right superior, 11 in the left inferior and 6 in the right inferior PV. 4 foci were found in the atrium: 3 in the right atrium and 1 in the posterior left atrium. RF catheter ablation of the triggering foci was undertaken. A single session resulted in successful ablation of the foci in 14 patients, 25 patients required two sessions, and 6 patients required three. At a mean follow-up 8 ± 6 months AF was completely eliminated in 28 patients (62 %) without drug therapy.
As a result, an initial strategy for catheter ablation was to induce AF triggers, map, and ablate the triggers within the PVs. This strategy was limited by the inability to induce AF triggers in up to 1/3 and a high recurrence rate after ablation. Freedom from recurrence of AF at 14 months without drugs was 33 %. An additional 13 % had no AF, but remained on drugs. PV ablation led to PV stenosis in up to 38 %. While treatment of PV stenosis with stenting was possible, a better approach was needed to improve success and decrease complications.
To avoid the need to induce, map and ablate the individual PV triggers, Pappone et al. in 2000, described the technique of PV isolation [12]. This technique was performed using a circular catheter with multiple electrodes on it that was positioned outside each of the PVs which isolated the triggers from the left atrium preventing any triggers inside the PVs from starting AF. Since 2001, the location of the PV isolation has moved more proximally into the left atrium, resulting in PV antral isolation [13]. PV antral isolation is now the standard for most patients with paroxysmal AF. A search for AF triggers outside the PV may be needed in other patients.
In patients with persistent and especially in long-standing AF, atrial remodeling becomes an important mechanistic factor for maintenance of AF [14] resulting in a high recurrence rate with PV antral isolation alone [14]. To achieve success, these patients often require a more complex ablation strategy which includes lines of linear ablation, ablation of non-PV triggers, ablation of complex fractionated atrial electrograms, extensive ablation of left atrial posterior wall, and/or ablation of the ganglionated plexi [14]. The resultant extensive scar formation can lead to possible adverse consequences in the left atrium [14].
Outcomes and Efficacy of Catheter Ablation
Well-designed large randomized multicenter trails have been the mainstay of cardiovascular research to evaluate outcomes and efficacy for cardiovascular disease for over 30 years. These data are presently lacking for AF ablation. The available data for AF ablation is largely limited to a small number of randomized studies with small sample sizes of <100 in each arm that studied younger patients (<60 years old) with few comorbidities and predominately paroxysmal AF with a short follow-up of a year or two. The end points were usually suppression of AF compared to antiarrhythmic drug therapy rather than the harder clinical end points of morbidity and mortality outcomes. Although the available data are less than ideal, catheter ablation is superior to antiarrhythmic drug therapy in maintaining NSR. Prior studies (AFFIRM [8] and RACE [9]) comparing rate control to rhythm control failed to show the superiority of rhythm control with antiarrhythmic drugs over rate control in most AF cases. Whether a strategy of rhythm control using catheter ablation will be the superior strategy remains unknown.
The most important predictor for success of catheter ablation is the type of AF being treated [12]. Paroxysmal AF has a significantly higher success rate for NSR maintenance than persistent AF which has a higher success rate than long-standing persistent AF. Similarly, variables such as age, concomitant cardiac disease, obesity, sleep apnea, and LA size impact outcome [12].
The 5 year follow up results of catheter ablation for maintaining NSR has been reported by Weerasooriya et al. [15]. The average age at inclusion was 55.7 ± 9.6 years, 64 % had paroxysmal AF, 22 % had persistent AF, and 14 % had long-standing persistent AF. Structural heart disease was present in only 36 %, with 16 % having LVH, and the LVEF was normal at 70 ± 11 %. The CHADS2 score was 0 in 48 %, 1 in 32 % and ≥2 in 20 % of the subjects. For this group of younger, generally healthier patients than the general AF population, freedom from recurrent AF at 5 years was 63 %. However, 51 % required repeat interventions. The 5 year results for freedom from recurrent AF with a single procedure was disappointing at only 29 % (Fig. 23.1). There was an 8.9 % gradual straight line annual recurrence rate of AF after the last ablation attempt over the 5 year follow-up period (Fig. 23.2). The 8.9 % annual recurrence rate after the last ablation attempt is consistent with results from other studies with “long-term” follow-up of 2–3 years. Based on these results, it appears that catheter ablation is not a cure but a treatment for AF that requires continued long term follow-up.
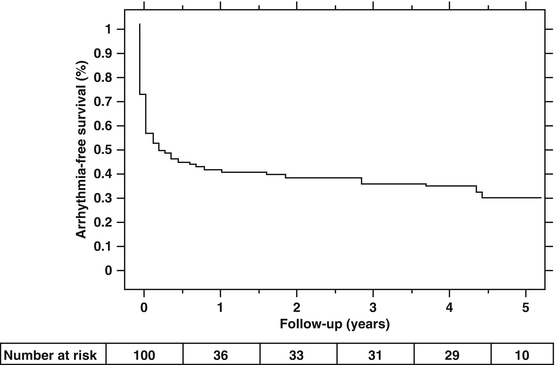
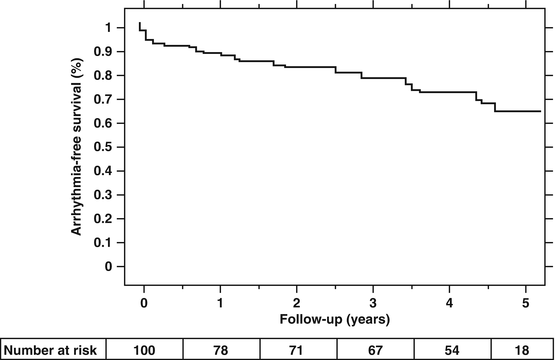

Fig. 23.1
Single Procedure Success. Kaplan-Meier event-free survival curve after a single catheter ablation attempt (Reproduced from Weerasooriya et al. [15], with permission of Elsevier)

Fig. 23.2
Multiple Procedure Success. Kaplan-Meier event-free survival curve after the last catheter ablation attempt (Reproduced from Weerasooriya et al. [15], with permission of Elsevier)
In a recently reported randomized trail comparing radiofrequency ablation vs antiarrhythmic drugs as first-line treatment of paroxysmal AF (RAFFT-2), 61 patients in the antiarrhythmic drug group and 66 patients in the radiofrequency ablation group were followed for 2 years for recurrence and quality of life measures [16]. The patients had an average age of 55 years old, little comorbidity, an average LVEF of 61 %, a normal or mildly increased LA size, and a median CHADS2 score of 0. These patients do not represent the typical AF population. Recurrence of AF occurred in 72.1 % with antiarrhythmics and 54.5 % with ablation at 2 years follow-up. There was no difference in the quality of life measures between the groups at baseline and during the study at the 1 year follow-up. There was a 9 % rate of serious adverse events with ablation, with pericardial effusion and tamponade occurring in 6 % [16] (Table 23.2).
Table 23.2
Randomized trials of catheter ablation
RAAFT-2 (paroxysmal AF) < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|
|---|