When and How to Re-Ablate
Michael Riley
David Callans
Every electrophysiologist who performs successful ablations for atrial fibrillation (AF) also performs ablations that are unsuccessful, characterized by a recurrence of atrial arrhythmias. The rate of these unsuccessful AF ablations is significant. Using simple subtraction and published success rates that, in broad terms, range from 50% to 90% for initial AF ablations, the recurrence rate is by definition 10% to 50%, a figure that may be higher when performed outside of referral centers with highly experienced operators. As any experienced practitioner of AF ablations can attest, these percentages are not trivial in number or in the effort required to support, treat, and follow-up these patients. Many, if not most of the patients who have recurrence of atrial arrhythmias following their initial procedure will undergo at least a second ablation procedure. Despite the wealth of data and hard work devoted to understanding the optimal initial strategy for ablating AF, relatively little has been devoted to understanding the re-do ablation procedure.
This chapter is devoted to re-do AF ablations focusing on issues such as the nature of recurrent arrhythmias, patient selection, preprocedure monitoring and planning, unique challenges of re-do ablations, and what one can expect to find and deal with during the ablation, as well as during follow-up following ablation.
Published success and recurrence rates following initial AF ablation, describing the successes and failures of highly experienced operators at highly experienced referral centers, vary tremendously (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12). The differences reflect definitional differences about what constitutes “success,” including how non-AF arrhythmias are categorized, the patient population studied, the extent of follow-up monitoring, the use of antiarrhythmic drugs, the role of repeat ablation procedures, and potential differences in the effectiveness of the different strategies. How the published recurrence rates translate into those ablations performed by the general electrophysiology (EP) community is largely unknown, though data from a world-wide survey of AF
ablation suggest higher recurrence rates when AF ablation is performed at centers performing fewer ablations per year (13). In addition, there continues to be an ongoing evolution regarding the “best” strategy for the procedure, due in part to an ongoing expansion of concepts and theories related to our understanding of AF and AF ablation, including interest in gangliated plexi, regions of complex fractionation, and others.
ablation suggest higher recurrence rates when AF ablation is performed at centers performing fewer ablations per year (13). In addition, there continues to be an ongoing evolution regarding the “best” strategy for the procedure, due in part to an ongoing expansion of concepts and theories related to our understanding of AF and AF ablation, including interest in gangliated plexi, regions of complex fractionation, and others.
Following AF ablation, several different atrial arrhythmias may occur and even coexist in the same patient (Table 20.1). The most frequent recurrent arrhythmia is AF, particularly in initial procedures that are focused on pulmonary vein isolation (PVI). Diagnosis is usually straightforward, based on office ECGs and/or ambulatory monitoring with or without patient symptoms. Recurrent AF following ablation can have important differences compared with the AF experienced prior to ablation. That is, previously ineffective antiarrhythmic medications may now become effective. Recurrent AF episodes may become more infrequent, shorter in duration, or even less symptomatic. These differences can be significant enough that many authors consider a 90% reduction in the frequency of symptomatic AF to constitute a successful outcome. Although the durability of this endpoint is not clear, some patients with AF control with or without medications will have progressive increase in AF burden over time.
Four other types of atrial arrhythmia have also been observed following AF ablation: typical atrial flutter (macroreentrant circuit utilizing the cavotricuspid isthmus), atypical macroreentrant flutter, focal reentrant atrial tachycardia, and focal nonreentrant atrial tachycardia. We have observed that these arrhythmias can be quite symptomatic, often more so than AF due in part to the faster ventricular rate that often accompanies these rhythms. Although the pathogenesis of these arrhythmias is not completely clear, typical and atypical flutter are consistently associated with recurrent atrial-PV conduction. We assume that these rhythms are initiated by PV triggers and some attention should be directed to repeat PVI during repeat procedures for these arrhythmias as well.
Typical atrial flutter often coexists with AF. There is some evidence that PVI can effectively, though not completely, treat both AF and preexisting typical atrial flutter (14). We and others have adopted the practice of performing ablation to the cavotricuspid isthmus if there is evidence for pre-existing flutter or if typical flutter is induced during the AF ablation procedure (15). Regardless of whether typical flutter was apparent clinically prior to AF ablation, it can present de novo following AF ablation. Kilicaslan et al. have shown that typical flutter appearing after AF ablation may be more common in patients who have undergone prior cardiac surgery (14).
TABLE 20.1 Atrial Tachyarrhythmias Following AF Ablation | |
|---|---|
|
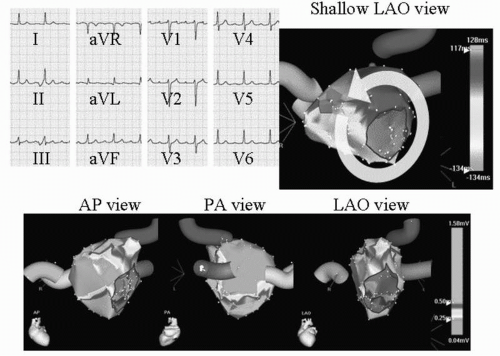
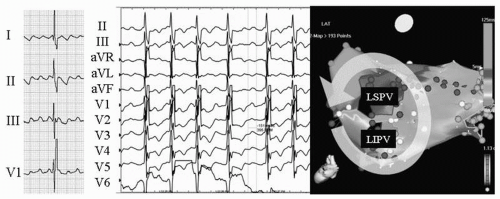
The occurrence of atypical macroreentrant flutter following AF ablation may have some correlation with the type of strategy employed, in particular with the use of wide circumferential lesion sets with adjunctive linear lesions. Most of these flutters appear to involve the left atrium, utilizing anatomic barriers (mitral valve, PV ostium), scar from ablation lesions, and gaps in linear ablation lines to produce the necessary components for the circuits. Though several different macroreentrant circuits have been observed following AF ablation, they commonly involve the mitral isthmus—the region between the left inferior PV and the mitral annulus (16, 17, 18, 19). An example of mitral annular flutter is shown in Figure 20.1. The extensive areas of low bipolar voltage in the left atrium shown in Figure 20.1 likely facilitate the maintenance of the macroreentrant flutter circuit. Figure 20.2 is an example of a different left atrial flutter circuit that was also dependent on the mitral isthmus. The interatrial septum, left atrial roof, gaps in previous ablation lines, and coronary sinus have also been observed to serve as critical isthmuses for atypical flutters (16,17,20).
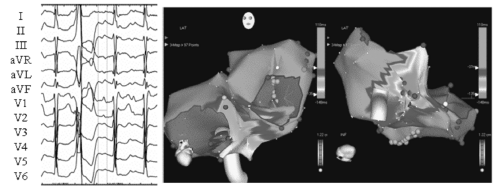
Focal atrial tachycardias—either reentrant or nonreentrant—have also been found to occur and it appears that they arise predominantly from PVs and the coronary sinus (20, 21, 22). Figure 20.3 is an example of a PV atrial tachycardia observed following PVI. This was detected with both ambulatory monitoring and ECGs recorded during highly symptomatic episodes. Figure 20.4 is an example of a non-PV atrial tachycardia that became manifest several years following initial ablation. The variety of atrial arrhythmias that can coexist in patients following AF ablation underscores the importance of investigating and documenting all arrhythmias a patient may be experiencing, both symptomatic and asymptomatic, so that all of the re-ablating that needs to be performed is performed during the re-do ablation. Preprocedural ambulatory monitoring is essential in this regard (see below). In addition, it is our practice to perform programmed atrial stimulation during any AF ablation procedure, and to characterize and target for ablation those sustained and organized atrial rhythms with cycle lengths ≥240 ms.
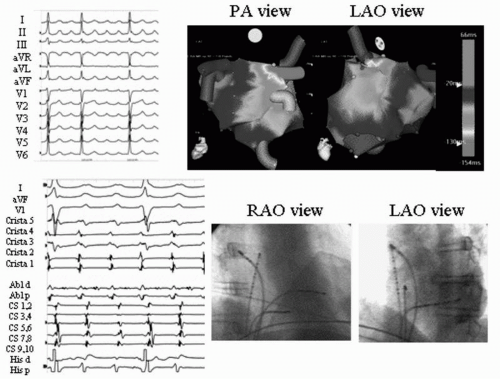 Figure 20.3. Focal atrial tachycardia arising from the right superior PV following PVI. The patient was undergoing re-do ablation for recurrent paroxysmal AF and highly symptomatic atrial tachycardia following initial ablation utilizing segmental PVI. Top left: 12-lead ECG of atrial tachycardia morphology. Top right: Biatrial activation maps of atrial tachycardia. Bottom left: Mid-diastolic potential on distal ablator at site of successful ablation. Bottom right: Biplane fluoroscopic images at site of successful ablation. See color insert 2. |
Despite the differences regarding strategy for ablation, a growing body of relatively consistent data suggests that some patients are more likely to recur than others (6,7,23, 24, 25, 26). Table 20.2 lists the set of patient characteristics that appear to correlate with a higher likelihood of recurrence of atrial arrhythmias following AF ablation.
Unfortunately, some of these characteristics are often correlated with occurrence of AF, and improvements in ablative therapy for patients such as those with persistent AF in the setting of structural heart disease represent a frontier in AF ablation.
Unfortunately, some of these characteristics are often correlated with occurrence of AF, and improvements in ablative therapy for patients such as those with persistent AF in the setting of structural heart disease represent a frontier in AF ablation.
TABLE 20.2 Predictors of Atrial Arrhythmia Recurrence Following AF Ablation | |
|---|---|
|
Recurrent episodes of AF tend to be catalogued as early (≤4-8 weeks), late (≤1 year), and very late (>1 year). It in now clear that early recurrence, though it is associated with, is not necessarily predictive of long-term failure; and most practitioners have adopted a strategy to treat early recurrences aggressively with medications and cardioversions. This approach is supported by the recently developed expert consensus guidelines suggesting that repeat ablation be deferred until at least 3 months have elapsed given the potentially transient nature of early recurrent arrhythmias (8) (and see section below, “Timing Re-Ablation”).
The subset of patients who recur very late (>12 months) following initial ablation may represent a unique population (27,28). Mainigi et al. performed a detailed characterization of patients who recurred greater than 12 months following their initial ablation and found that they tended to be more obese, had fewer PVs isolated, and had more non-PV triggers (28). In addition, during re-do ablation, these patients were found to have chronic reconnection of PVs and new triggers for AF.
Given the increasing maturity of ablation procedures for AF, we are now seeing patients who had undergone successful procedures 3 or more years ago returning with either newly “reemergent” AF or a relatively abrupt worsening of prior control of their AF. Very little is known about these patients in terms of electrophysiologic findings on reablation. The role of new or old triggers of AF or the relevance of changes in the atrial substrate is not understood.