Stent
Drug (concentration)
Polymer
Polymer thickness (μm)
Release kinetics (days)
Metal
Geometry
Strut thickness (μm)
First generation stents
CYPHER
Sirolimus (140 μg/cm2)
Polyethelyne co-vinyl acetate & PBMA
12.6
80 % (28)
SS
Closed cell
140
TAXUS express
Paclitaxel (100 μg/cm2)
Poly(styrene-b-isobutylene-b-styrene)
16
<10 % (28)
SS
Open cell
132
TAXUS liberté
Paclitaxel (100 μg/cm2)
Poly(styrene-b-isobutylene-b-styrene)
16
<10 % (28)
SS
Hybrid
97
Second generation stents
TAXUS element
Paclitaxel (100 μg/cm2)
Poly(styrene-b-isobutylene-b-styrene)
15
<10 % (90)
PtCr
Open cell
81
Endeavor
Zotarolimus (100 μg/cm2)
Phosphorylcholine
4.1
95 % (14)
CoCr
Open cell
91
Endeavor RESOLUTE
Zotarolimus (10 μg/mm)
Biolinx
4.1
85 % (60)
CoCr
Open cell
91
Xience V
Everolimus (100 μg/cm2)
PBMA & PVDF-HFP
7
80 % (90)
CoCr
Open cell
81
PROMUS element
Everolimus (100 μg/cm2)
PBMA & PVDF-HFP
7
80 % (90)
PtCr
Open cell
81
Table 21.2
Rates of death, myocardial infarction, target lesion revascularization and stent thrombosis from meta-analyses of drug eluting stents compared to bare metal stents
Reference | Type of meta-analysis | Number of patients (DES/BMS) | Longest follow-up (years) | Death (DES vs. BMS) | MI (DES vs. BMS) | TLR (DES vs. BMS) | Definite ST (DES vs. BMS) |
|---|---|---|---|---|---|---|---|
Stettler et al. [10]a | Collaborative network | 18,023 (13,102/4921) | 4 | HR 0.96 | HR 0.83* | HR 0.70* | cHR 0.95 to HR 1.02 |
dHR 1.14 to HR 1.61 | |||||||
eHR 1.43 to HR 3.57 | |||||||
Kirtane et al. [9] [on-label] | Comprehensive | 9470 (4867/4603) | 5 | HR 1.05 | HR 1.03 | HR 0.54* | |
Kirtane et al. [9] [off-label] | HR 0.84 | HR 0.83 | HR 0.42* | ||||
Kang [11]a,b | Bayesian approach network | 90,584 (80,744/9844) | 5 | HR 0.62 to HR 0.87 | HR 0.42* to HR 0.85 | HR 0.19* to HR 0.43* | c,dHR 0.25* to HR 0.84 |
eHR 0.23* to HR 2.13* | |||||||
Bangalore [12]b | Mixed treatment comparison | 106,427 | 5 | RR 0.69 to RR 0.89 | RR 0.61* to RR 0.98 | RR 0.30* to RR 0.57* | c,d,eRR 0.35* to RR 1.17 |
Despite the above data, BMS continue to have a role, albeit limited, in clinical practice. Some of the cited reasons for using BMS [17] can be refuted with contemporary data, which have failed to demonstrate similar efficacy between BMS and DES for large vessel intervention, [18] or an increased risk of ST with DES compared to BMS in primary PCI for ST-elevation MI [15]. Other factors influencing the decision include patient age, and cost/reimbursement. Overall, however, the commonest cited reason to use a BMS appears to be conflict related to the use of dual anti-platelet therapy (DAPT) be it the risk of bleeding from prolonged use due to co-morbidities, or its anticipated premature interruption due to suspected poor compliance or planned non-cardiac surgery. Use of a BMS in these scenarios may be considered more appropriate as premature interruption of DAPT is an important risk for ST, [19, 20] and implantation of a BMS mandates only 1 month of DAPT, as opposed to 6–12 months for DES [21]. As a caveat however, there are emerging data, which confirm the safety of using shorter durations of DAPT with DES, [22–25]. More information will be available in the future. The currently recruiting Global Leaders Study includes one arm where patients treated with a DES receive only 1 month of DAPT.
Which Anti-proliferative Agent?
The previous discussions have established the clear clinical benefits of DES compared to BMS, however which anti-proliferative drug should be eluted? The role of this agent is ultimately to limit neointimal proliferation, and consequently its ideal properties should include the following: a wide therapeutic window, a low inflammatory potential, a select ability to suppress smooth muscle cell proliferation without being toxic to the medial and adventitial cell layers and an ability to promote re-endothelialization.
Throughout the DES era two main classess of drugs have been used (Fig. 21.1): (1) immuosupressant limus analogues which function by inhibiting the mammalian target of rapamycin (mTOR), thereby reversibly inhibiting growth factor and cytokine stimulated cell proliferation in the G1 phase of the cell cycle, and (2) anti-proliferative paclitaxel which inhibits cell replication predominantly in the G0/G1 and G2/M phases of the cell cycle. The demonstrated superiority of limus-eluting DES compared to paclitaxel in randomized studies and subsequent meta-analyses in terms of significantly lower late lumen loss, repeat revascularizations, and ST [11, 12, 26] has largely led to paclitaxel losing its role in contemporary DES. However, its properties have resulted in it being the anti-proliferative agent of choice to be released from drug-coated balloons [27].
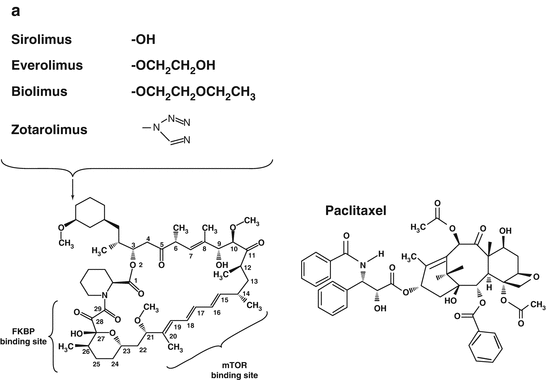
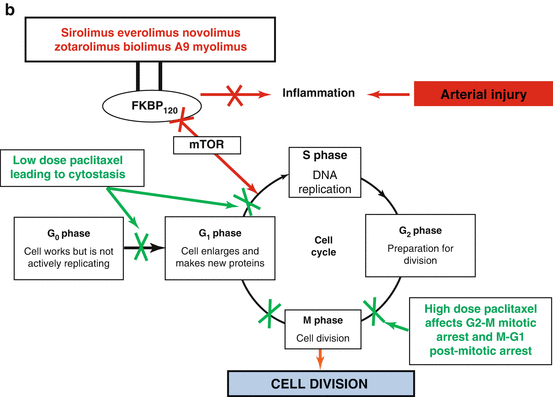


Fig. 21.1
The chemical structure (a) and mechanism of action (b) of the macrocyclic lactone group of anti-proliferative drugs and paclitaxel
It follows that contemporary DES elute macrocyclic lactones, which are similar to sirolimus. However, modifications on the carbon atom 40 of the macrocyclic ring have lead to the development of zotarolimus, everolimus, and biolimus. Other agents such as novolimus have been produced through removal of a methyl group from carbon atom 16, and myolimus through replacement of the oxygen on carbon atom 32 of the macrocyclic ring. These agents all offer subtle differences in degrees of immunosuppression and liphophilicity, with the latter influencing the rate of drug absorption into the arterial wall. The direct influence of these different agents on stent performance is impossible to evaluate in isolation, as concurrent modifications to other aspects of stent design have also been made. Pre-clinical data comparing stents with identical platforms, polymers, drug loads and drug release kinetics but releasing everolimus, sirolimus, or zotarolimus—demonstrate that all three limus drugs have comparable effects on neointimal suppression [28]. Clinical data are available from numerous randomized studies and their subsequent meta-analyses, comparing sirolimus-, everolimus-, zotarolimus- and biolimus- eluting stents individually with either BMS or other DES, with additional data obtained from indirect comparisons between these DES from network meta-analyses [10–12, 14, 16].
Conclusions from the largest network meta-analysis by Bangalore et al. [12] which included data from 126 trials and 258,544 patient years of follow-up suggest that the thin-strut, fluoro-polymer coated everolimus-eluting stent (EES, Table 21.1) has the best combination of efficacy and safety as evidenced by:
1.
EES being the only DES to show a significant reduction in mortality compared to BMS (HR 0.72, 95 % CI 0.58–0.90). No significant differences in mortality were seen in comparisons between different DES.
2.
EES having the greatest reduction in the risk of MI compared to BMS (HR 0.61, 95 % CI 0.44–0.87). In comparison, other limus DES reduce the risk of MI versus BMS with hazard ratios of 0.71–0.83. No significant differences in mortality were seen in comparisons between different DES.
3.
EES being the only DES to significantly reduce the rate of definite ST compared to BMS (HR 0.35, 95 % CI 0.21–0.53). A subsequent analysis demonstrated, with an 81 % probability, that EES was associated with the lowest rate of definite ST compared to other DES.
4.
EES has the greatest reduction in target vessel revascularization compared to BMS (HR 0.37, 95 % CI 0.26–0.52). In comparison, other limus DES reduce the risk of target vessel revascularization versus BMS with hazard ratios of 0.38–0.59. No significant differences in mortality were seen in comparisons between different DES.
The cobalt chromium EES stent has a strut thickness of 81 μm, and is coated with a 7.6 μm thick, non-erodable, co-polymer of poly vinylidene fluoride co-hexafluoropropylene (PVDF-HFP), and poly n-butyl methacrylate (PBMA), which facilitates elution of everolimus over 120-days. Everolimus (C53H83NO14, molecular weight 958 Da) is a sirolimus derivative, in which the hydroxyl group at position C40 of sirolimus has been alkylated with a 2-hydroxy-ethyl group. It is slightly more lipophilic than sirolimus, and therefore, it is more rapidly absorbed into the arterial wall. Although binding of everolimus to the FKBP-12 domain is three-fold and immunosuppressive activity in vitro two to five-fold lower than with sirolimus, oral everolimus proved at least as potent as sirolimus in models of autoimmune disease and heart transplantation. The EES platform is potentially associated with less inflammation than SES and PES [29]. The thin-strut structure of the stent platform, the thromboresistant properties of the fluoro-polymer, and the reduced polymer and drug load may contribute to the low rate of ST with EES [30]. The notion of a DES being safer than a BMS represents a paradigm shift in the evolution of PCI.
Stent Polymer
The role of the stent polymer is to facilitate controlled elution of anti-proliferative drugs over a specified period of time, with their presence obsolete once drug elution has been completed. This latter fact, together with evidence identifying polymers, particularly the non-biocompatible polymers found on first generation DES as a nidus for chronic inflammation within the arterial wall leading to hypersensitivity reactions, endothelial dysfunction and subsequent delayed healing, has led to accusations that they are central to triggering late and very late ST [29, 31–33]. Valid questions have therefore been raised regarding whether the polymer should be permanent or biodegradable, (i.e. biodegrade once drug elution has been completed), or whether they are needed at all.
Permanent Versus Biodegradable Polymer
In theory, stents with biodegradable polymers offer the anti-restenotic benefits of conventional DES during vascular healing. However, once drug elution has been completed and the polymer has broken down, the stent should offer the safety benefits of a BMS. While this concept is attractive, there remain several challenges including (1) establishing the optimal biocompatibility, composition, formulation, and degradation time of the polymer; (2) identifying the optimal pharmacokinetics of the anti-proliferative agent released by the degradation of the polymer; (3) managing variations in polymer degradation time which can be affected by production factors such as the use of long polymer chains, decreased polymer hydrophobicity and greater polymer crystalinization and biological environmental factors; [34] (4) dealing with the potential complications of an inflammatory and immune reaction to polymer breakdown [35].
Despite these conceptual challenges, numerous DES utilizing biodegradable polymers, such as poly-lactic acid, poly-L-lactic acid (PLLA), and poly(D,L-lactide-co-glycolide), have been developed and undergone clinical evaluation in first-in-man studies, and randomized clinical studies with BMS and/or permanent polymer DES as the comparator arm (Table 21.3) [18, 36–58]. Reassuringly, porcine studies have shown less inflammation, [59] and clinical studies, improved vasomotion, [60, 61] and fewer uncovered struts as assessed by optical coherency tomography at 6–8 months follow-up [62] with biodegradable versus permanent polymer DES. Unfortunately however, proof-of-concept remains to be reliably established as only few studies are available with sufficiently long enough follow-up to examine whether these devices truly offer an advantage in terms of long-term clinical safety [63]. One such study is the LEADERS study, which showed a significantly lower rate of very late definite ST between 1- and 5-years follow-up with the BioMatrix (Biosensor, Morges, Switzerland), biodegradable polymer biolimus-eluting stent (BES) compared to Cypher permanent polymer SES (Fig. 21.2) [63].
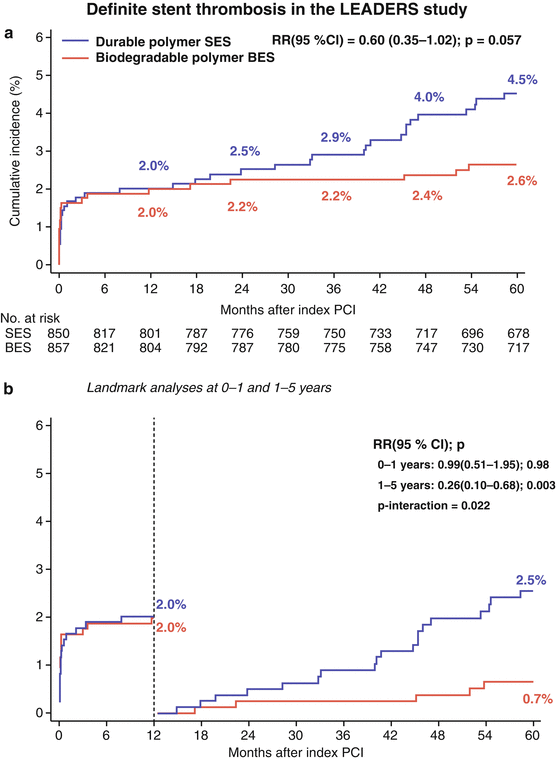




Table 21.3
Metallic stents with a biodegradable polymer which are either currently available or undergoing clinical evaluation
Stent (manufacturer) (Ref) | Drug (dosage) | Drug release (%) release/time | Stent platform | Strut/max coating thickness (μm) | Polymer type (duration of biodegradation, months) | In-stent late loss (mm) |
|---|---|---|---|---|---|---|
Supralimus (Sahajanand Medical) [43] | Sirolimus (125 μg/19 mm) | 50 % | SS | 80/4–5 | PLLA, PLGA, PLC, PVP (7) | 0.09* |
9–11 days | ||||||
Excel stent (JW Medical System) [44] | Sirolimus (195–376 μg) | NA | SS | 119/15 | PLA (6–9) | 0.21* |
FIREHAWK (MicroPort) [45] | Sirolimus (55 μg/18 mm) | 75 % | CoCr with grooves | NA | Abluminal groove filled PLA (9) | 0.13§ |
30 days | ||||||
MiStent (Micell) [46] | Crystalline Sirolimus (9–11 μg/mm) | 100 % | CoCr | 64/15 | PLGA (3) | 0.03§ |
60 days | ||||||
Biolimus A9 (15.6 μg/mm) | 45 % | SS | 112/10a | Abluminal PLA (6–9) | 0.13† | |
30 days | ||||||
NOBORI (Terumo) [48] | Biolimus A9 (15.6 μg/mm) | 45 % | SS | 112/10a | Abluminal PLA (6–9) | 0.11† |
30 days | ||||||
Axxess (Biosensors) [49] | Biolimus A9 (22 μg/mm) | 45 % | Nitinol | 152/15a | Abluminal PLA (6–9) | 0.29 MB† |
30 days | 0.29SB† | |||||
SYNERGY (Boston Scientific) [37] | Everolimus (LD 56 μg/20 mm) (SD 113 μg/20 mm) | 50 % | PtCr | 71/3 (LD) | PLGA Rollcoat Abluminal (3) | 0.13 (LD)* |
60 days | 4 (SD) | 0.10 (SD)* | ||||
Combo (OrbusNeich) [50] | EPC + Sirolimus (5 μg/mm) | 95 % | SS | 100/3–5a | Abluminal PLA, PLGA, CAP(<3) | 0.39† |
35 days | ||||||
DESyne BD (Elixir Medical) [51] | Novolimus (5 μg/mm) | 90 % | CoCr | 81/<3 | PLA (6–9) | 0.16* |
90 days | ||||||
Elixir Myolimus (Elixir Medical) [52] | Myolimus (3 μg/mm) | 90 % | CoCr | 81/<3 | PLA (6–9) | 0.08* |
90 days | ||||||
Svelte (Svelte Medical) [53] | Sirolimus (220 μg/cm2) | 80 % | CoCr | 81/6 | PLGA (12) | 0.22* |
28 days | ||||||
BioMime (Meril Life Sciences) [54] | Sirolimus (1.25 μg/mm2) | NA | CoCr | 65/2 | PLGA + PLLA | 0.15^ |
Orsiro (Biotronik) [55] | Sirolimus (1.4 μg/mm2) | 100 % | CoCr | 71/11 | PLLA (15) | 0.05† |
100 days | ||||||
Inspiron DES (Scitech) [56] | Sirolimus (56 μg/13 mm) | NA | CoCr | 75/5a | PLA + PLGA (6–8) | 0.22* |
Paclitaxel (122 μg/19 mm) | 50 % | SS | 80/4–5 | PLLA, PLGA, PLC PVP (7) | 0.54† | |
9–11 days |

Fig. 21.2
The cumulative incidence (a) and landmark analyses (b) of definite stent thrombosis for patients receiving the biodegradable polymer biolimus-eluting stent versus the permanent polymer sirolimus-eluting stent in the all-comers randomized LEADERS study [63]. The rates of stent thrombosis were comparable for both stents at 1-year, and significantly lower with the biodegradable polymer stent between 1 and 5 years follow-up
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


