Chapter 32 Water and Electrolyte Disturbances
Disturbances in water and electrolytes are common in the intensive care unit (ICU). In the first section of this chapter the physiology of water balance, including the distribution of intravenous fluids and the effects of cardiopulmonary bypass (CPB), are briefly reviewed. In the second section, clinical abnormalities of water and electrolytes are discussed. Common terms are explained in Table 32-1. Relevant physiology discussed in Chapter 1 is referred to throughout.
Table 32-1 Definitions of Terms
| Osmosis | The movement of water caused by a concentration difference in the water on each side of a semipermeable membrane |
| Osmotic pressure | The hydrostatic pressure that must be applied to stop osmosis |
| Osmole | The number of molecules present in 1 g of a substance, multiplied by its molecular weight (of undissociated solute) |
| Performing this calculation allows the concentration of a solute to be expressed in terms of the number of molecules. For example, if 60 g urea ([NH2]2CO, molecular weight = 60 g) is dissolved in 1 kg of water, the osmolality of the solution is 1 osmole per kg. For a substance that fully dissociates (e.g., NaCl), if the molecular weight (in grams) is dissolved in 1 kg of solute, the concentration is 2 osmoles per kg. | |
| Osmolality | The number of osmoles per kilogram of water |
| Osmolarity | The number of osmoles per (total) liter of solution |
| When solute is dissolved in solvent, the volume is greater than the volume of the solvent alone. | |
| In dilute solutions with small solute molecules, osmolarity is (approximately) equal to osmolality. | |
| In concentrated solutions of large solvent molecules, osmolality is greater than osmolarity. | |
| Tonicity | The “effective” osmolality |
| Solutes that are distributed evenly across semipermeable membranes do not exert osmotic pressure. | |
| Solutes that are restricted in their movement across compartments are “effective osmoles,” and tonicity describes their concentration. |
PHYSIOLOGY
Body Water: Distribution, Constituents, and Movement
The intracellular and interstitial (extracellular) compartments are separated by cellular membranes. Water distributes freely between these two compartments according to the osmotic pressure difference across cellular membranes. At equilibrium, the number of osmotically active particles inside and outside cells is equal (about 285 mOsm/l). However, the constituents of the intracellular and extracellular fluids are quite different (Table 32-2). Some substances diffuse freely across cell membranes (e.g., oxygen, carbon dioxide, urea) and are therefore at the same concentration inside and outside cells. The movement of other substances (notably ions and glucose) across cell membranes is tightly controlled by selective permeability and active transport, which results in different concentrations inside and outside cells (see Table 32-2). The major cation in the extracellular fluid is sodium, and under normal circumstances it is the primary determinant of plasma osmolarity. The major anions in the extracellular fluid are chloride and bicarbonate. Because ions carry positive and negative charges, the active transport and selective permeability of ions allows variable charge separation to occur across the cell membrane, leading to the formation of resting and active membrane potentials (see Chapter 1).
Table 32-2 Composition of Intracellular and Extracellular Fluid
| Electrolyte | Intracellular Fluid (mmol/l) | Extracellular Fluid* (mmol/l) |
|---|---|---|
| Sodium | 10 | 140 |
| Potassium | 155 | 3.8 |
| Chloride | 3 | 108 |
| Bicarbonate | 10 | 26 |
| Calcium (ionized) | <0.01 | 1.2 |
| Magnesium | 10 | 0.8 |
| Phosphate | 105 | 1.0 |
* Because the plasma contains highly negatively charged protein molecules, minor differences exist between the electrolyte content of the interstitial fluid and the plasma.
In addition to diffusion and osmosis, there is mass movement (filtration) of fluid between the capillary and the interstitium based on the balance of Starling forces across the capillary wall. The Starling forces are composed of the hydrostatic and the oncotic pressure gradients between the capillary lumen and the interstitial space (see Equation 1-12). The balance of the Starling forces varies among capillary beds, but overall there is a net loss of fluid from plasma to the interstitium. Filtered fluid is returned to the plasma through the lymphatic system.
Intravenous Fluids
Intravenous fluids may be crystalloids (Table 32-3) or colloids. They are used mainly for treating hypovolemia (resuscitation fluid) and for replacement of obligatory daily losses (maintenance fluid). They are administered intravenously (i.e., into the plasma space) and, depending on their constituents and tonicity, the water is distributed to a variable extent throughout the extracellular and intracellular compartments.
Crystalloids
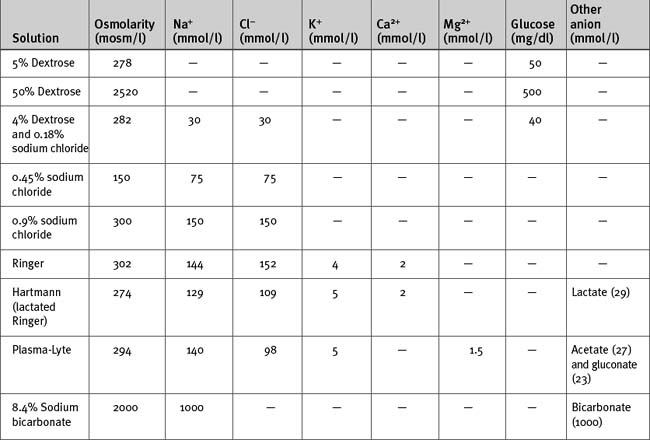
The anion in sodium-based crystalloids is predominantly chloride. Chloride is the only anion in 0.9% sodium chloride and Ringer solution and is therefore present in supraphysiologic concentrations. Large volumes of these solutions can cause a hyperchloremic metabolic acidosis (see Chapter 31). More physiologic concentrations of chloride are present in buffered, balanced electrolyte solutions such as Hartmann (lactated Ringer) and Plasma-Lyte. Because bicarbonate is not stable in solution for long periods, these solutions contain lactate (Hartmann) or acetate and gluconate (Plasma-Lyte) as the nonchloride anion.
Hypotonic sodium solutions reduce the tonicity of the extracellular fluid and therefore, by osmosis, some water will be distributed to the intracellular space. For instance, with 0.45% sodium chloride, which has an osmolarity of 150 mOsm/l, about half of the water will be distributed to the intracellular compartment. In contrast, hypertonic sodium solutions increase the tonicity of the extracellular fluid, and “drag” water from the intracellular compartment by osmosis. Thus, the extracellular compartment will expand by a volume greater than the volume administered. Hypotonic and hypertonic solutions are used in special circumstances. For instance, 0.45% sodium chloride is used to replace hypotonic renal losses during the polyuric phase of acute renal failure, whereas hypertonic sodium chloride is used to reduce intracranial pressure in patients with cerebral edema. Rapid administration of large volumes of hypotonic solutions (e.g., pure water) into a peripheral vein (where flow may be sluggish) may cause osmotically mediated swelling—and potential lysis—of red blood cells. Ideally these solutions should be administered slowly via a central line.
Colloids
Colloid solutions contain large, oncotically active molecules in a base solution of either 0.9% sodium chloride or a buffered, balanced electrolyte solution. Colloid molecules are too big to traverse gap junctions, so more of the water in these solutions tends to be retained within the plasma space. Theoretically, assuming a plasma volume of 3 liters in a 70-kg patient, 1 liter of an isooncotic colloid solution increases plasma volume by 1 liter, which is 4 to 5 times the plasma volume expansion achieved by the same volume of an isotonic sodium-based crystalloid. However, with critical illness, the vascular endothelium “leaks,” allowing colloid molecules to pass into the interstitium, where they exert an osmotic pressure effect. This probably explains the observation that when resuscitating critically ill patients with 0.9% sodium chloride only 1.3 times the volume is required (not four times as predicted) compared with 4% albumin to achieve the same hemodynamic end points.1
Commonly used natural colloids include albumin and fresh-frozen plasma. Albumin is available as 4%, 5%, and 20% preparations. Both 4% and 5% solutions are approximately isooncotic with plasma; 20% albumin is hyperoncotic and therefore expands the plasma volume by about four times its volume. Commonly used artificial colloids include modified gelatins (e.g., Gelofusine) and hydroxyethyl starch compounds. Hydroxyethyl starch comprises a family of colloids that are categorized on the basis of their average size into high (>400 kD), medium (200 kD), and low (70 kD) molecular weight preparations.2 Two commonly used hydroxyethyl starches are hetastarch (average molecular weight 480 kD) and pentastarch (average molecular weight 200 kD). By comparison, Gelofusine has an average molecular weight of about 35 kD. Most artificial colloids are isooncotic or slightly hyperoncotic. The plasma half-time of artificial colloids varies among preparations but is typically on the order of 4 to 6 hours. Thus, the colloid effect has largely dissipated by 24 hours. Large volumes of hydroxyethyl starch can cause impaired hemostasis, mainly because of reduced effectiveness of the factor VIII/von Willebrand factor complex (i.e., acquired von Willebrand disease).2 High molecular weight compounds such as hetastarch appear to cause greater impairment of hemostasis than do medium-sized compounds such as pentastarch.3,4 Impaired hemostasis may also occur with gelatin-based colloids.5 To avoid hemostatic problems, the dosages of these artificial colloids should be kept below 20 ml/kg. All artificial colloids can cause allergic (including anaphylactic) reactions.
Crystalloids Versus Colloids for Fluid Resuscitation
There has been a great deal of debate over many decades on the pros and cons of crystalloids versus colloids for fluid resuscitation. Some ICUs use predominantly colloids, others predominantly crystalloids. Colloids are popular in Europe, whereas crystalloids are popular in North America. However, as long as fluids are administered to appropriate physiologic end points the choice between a colloid and a crystalloid is not important.1,6 In view of the additional cost and potential for adverse events with artificial colloids, a primarily crystalloid-based fluid regime seems preferable.
In 1998, a metaanalysis found an apparent excess mortality rate associated with the use of albumin in critically ill patients.7 This and a subsequent analysis by the same group8 created a storm of controversy and led to the publication of other metaanalyses that did not support the original finding.9,10 The issue of the safety of albumin was resolved in 2004 with the publication of a prospective, randomized trial involving nearly 7000 patients that found no difference in mortality rates between patients resuscitated with 4% albumin and those given 0.9% sodium chloride.1
Regulation of the Osmolarity and Volume of the Extracellular Compartment
Regulation of Water Balance
In addition to increased osmolarity, antidiuretic hormone secretion is also stimulated by hypotension and hypovolemia. In the presence of two competing pathophysiologic states—hypovolemia and low tonicity— circulating volume is defended over tonicity.