Study
Population
N
Male (%)
Age (years)
Baseline 25(OH)D (ng/mL)
Follow-up (years)
Exposure ([25(OH)D] in ng/mL)
Outcome
Association
Wang et al. [10]
Community-dwelling adults, no previous CVD
1,739
45
59
19.7
5.4
25(OH)D <15 vs. 25(OH)D ≥15
MI, angina, Stroke, TIA, HF
HR 1.62 (95 % CI 1.11–2.36)
Giovannucci et al. [11]
Men between 40 and 75, no current CVD
18,225
100
64
23.6
10
25(OH)D <15 vs. 25(OH)D >30
Nonfatal MI, fatal CHD
RR 2.42 (95 % CI 1.53–3.84)
Sun et al. [12]
Community-dwelling adults, free of CVD and cancer
118,864
38
n/a
n/a
21
Intake of 25(OH)D ≥600 IU/day vs. intake <100 IU/day
CHD and stroke
RR 0.84 (95 % CI 0.72, 0.97) for men; RR 1.02 (95 % CI 0.89, 1.17) for women
Brøndum-Jacobsen et al. [13]
Community dwelling adults without vitamin D fortified-diet.
10,170
44
57
17.6
29
25(OH)D <6 vs. 25(OH)D >24
Myocardial infarction
HR 1.64 (95 % CI 1.25–2.14)
Dobnig et al. [14]
Patients referred for coronary angiography
3,258
Stratified
62
Stratified
8
25(OH)D 13.3 vs. 25(OH)D 28.4
Cardiovascular death
HR 1.82 (95 % CI 1.29–2.58)
Wolf et al. [15]
Incident dialysis patients
825
53
63
21
90 days
25(OH)D <10 vs. 25(OH)D >30
Cardiovascular death
OR 1.9 (95 % CI 1.0–3.4)
van Ballegooijen et al. [16]
Community-dwelling older adults
256
Stratified
67
23
8
25(OH)D <14 vs. 25(OH)D >32
Change in LVMI
RC 9.9 (95 % CI 3.3, 16.6)
Fall et al. [17]
Elderly subjects, no previous heart disease
870
48
70
23.2
5
Baseline 25(OH)D
LVMI
B = –0.035 (95 % CI –0.915, 0.846)
Liu et al. [18]
Patients with heart failure
548
61
74
14.6
1.5
Decrease in [25(OH)D] by 4
HF hospitalization or death
HR 1.09 (95 % CI 1.00–1.16)
Pilz et al. [19]
Patients referred for coronary angiography
3,299
Stratified
Stratified
Stratified
8
25(OH)D <10 vs. 25(OH)D ≥30
HF or sudden cardiac death
HR 2.84 (95 % CI 1.20–6.74)
Forman et al. [20]
Community-dwelling adults without hypertension
1,809
Stratified
Stratified
Stratified
4
25(OH)D <15 vs. 25(OH)D ≥30
Incidence of hypertension
RR 3.18 (95 % CI 1.39 to 7.29)
Margolis et al. [21]
Postmenopausal women without hypertension
4,863
–
66
Stratified
7
Quartiles of 25(OH)D
Incidence of hypertension
Change in SBP or DBP by 25(OH)D quartile was not significantly different at any point in time
Observational Studies
Observational studies can assess the exposure (25(OH)D) and outcome (i.e. cardiovascular diseases) at the same point in time (cross-sectional) or they can assess the exposure and then outcome occurring after the exposure is measured (longitudinal). The cross-sectional studies are faster, cheaper and allow us to evaluate the relationship between the variables of interest at a given time point. However, the cross-sectional studies do not provide any information regarding the timing of the events as the longitudinal studies do. All observational studies are subject to confounding, which can be best minimized by randomization to intervention or placebo. In this section we will evaluate the data supporting the effects of vitamin D in cardiovascular diseases by study type.
Cross-Sectional Studies
A growing body of literature suggests that vitamin D insufficiency or deficiency may be associated with an increased risk of cardiovascular disease. However, the evidence thus far is inconclusive and subject to debate. Early studies reported a higher prevalence of hypertension in countries located in northern latitudes where populations are exposed to less sunlight than in countries nearer the equator [23, 24]. Similarly, large population studies such as the Third National Health and Nutrition Examination Survey (NHANES) identified cardiovascular risk factors (elevated blood pressure, plasma glucose and cholesterol) associated with lower 25(OH)D levels [25]. In contrast, an analysis of the Longitudinal Aging Study Amsterdam that included 1,205 participants patients older than 65 year-old did not find any association between 25(OH)D levels and blood pressure [26].
A second analysis of the NHANES database conducted by Melamed et al. included 13,331 free-living adults in the United States. These authors reported an increase in coronary artery disease (CAD) and stroke in patients with total 25(OH)D levels <20 ng/mL vs. those with 25(OH)D of >32 ng/mL [odds ratio (OR) 1.2 (95 % confidence interval (CI): 1.01–1.36)] [27]. Others have described an association between low 25(OH)D (<30 ng/mL) and heart failure (OR 1.7 [95 % 0.87–3.32]) in the same NHANES cohort [28]. In cases of extreme vitamin D deficiency (<15 ng/mL) in children (rickets), severe cardiomegaly and heart failure have been reported [29–33] Interestingly, patients with Vitamin D–resistant rickets as a result of congenital mutations in the VDR mostly die from cardiovascular diseases, but show no abnormalities in angiotensin converting enzyme activity, or in levels of angiotensin II and aldosterone [34].
Longitudinal Studies
Ischemic heart disease has been associated with extreme forms of vitamin D deficiency. For example in 1,739 participants of the Framingham Offspring Study, the risk of ischemic heart disease was associated with total 25(OH)D <15 ng/mL (hazard ratio [HR] of 1.62; 95 % CI 1.11–2.36, P = 0.01) compared to individuals with levels ≥15 ng/mL [10]. In a stratified analysis by diagnosis of hypertension at baseline, the association between 25(OH)D levels and ischemic heart disease was evident in patients with hypertension (HR 2.13, 95 % CI 1.30–3.48), but not in those without hypertension (HR 1.04, 95 % CI 0.55–1.96). Similar results were found in a study involving 18 225 men in the Health Professionals Follow-up Study [11] Furthermore, higher vitamin D intake from foods and supplements was associated with a decreased risk of cardiovascular disease including coronary artery disease and stroke in the Nurses Health Study and the Healthcare Professionals Follow- up Study [12]. Large global cohort studies have yielded similar findings. One such study of 10,170 women and men from the Danish general population showed that the risk for myocardial infarction was 64 % higher in participants with extreme 25(OH)D deficiency (<6 ng/mL) than participants with 25(OH)D >24 ng/mL [13]. Similarly, in the Ludwigshafen Risk and Cardiovascular Health (LURIC) study, both low 25(OH)D and 1,25(OH)2D levels were associated with cardiovascular mortality [14]. Of note, patients with extreme forms of chronic kidney disease who cannot convert 25(OH)D into the active hormonal form 1,25(OH)2D also have a high risk of cardiovascular mortality [15].
Studies evaluating the role of vitamin D in patients with left ventricular hypertrophy (LVH) show mixed results. An analysis of 256 participants from the Hoorn study, which is a population-based cohort in the Netherlands, demonstrated an association between low 25(OH)D and LVH only in individuals with history of cardiovascular disease and in those with low renal function (median estimated glomerular filtration rate ≤77.5 mL/min/1.73 m2) [16]. The effect appeared to be attenuated when adjusted for parathyroid hormone (PTH) levels. The Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS) study suggested an association between low 25(OH)D levels and altered left ventricular (LV) geometry and function at baseline (left ventricular end-systolic diameter and left ventricular ejection fraction), but failed to show the same association after 5 years of follow up [17]. In contrast, an analysis of 711 participants from the Baltimore Longitudinal Study of Aging revealed a relationship between 25(OH)D levels and left ventricular mass index (by echocardiogram), but did not find an association with left ventricular ejection fraction or geometry [35]. Similarly, a study that utilized cardiac magnetic resonance in 992 Icelandic community dwelling individuals found an association between 25(OH)D and left ventricular mass [36]. Populations with extreme forms of 25(OH)D deficiency such as the chronic kidney disease have a higher prevalence of LVH than patients with similar risk factors with normal renal function [37]. Heart failure patients with low 25(OH)D levels have a poor prognosis and higher inflammatory markers than those with higher 25(OH)D levels [18]. Furthermore, 25(OH)D levels are negatively correlated with natriuretic peptides and New York Heart Association class [19]. However, the relationship between vitamin D and heart failure is still controversial [38].
Blood pressure and vitamin D have been inconsistently linked in longitudinal studies. A combined analysis of the Health Professionals’ Follow-Up Study and the Nurses’ Health Study with 1,811 patients encompassing up to 8 years of follow-up reported a 2.67 fold increase in the relative risk of hypertension when comparing individuals with 25(OH)D <15 vs. >30 ng/mL [20] In contrast, the Women’s Health Initiative study reported no association between 25(OH)D and hypertension [21]. In the Norwegian Tromso study (n = 2,385), no difference in the incidence of hypertension was found in participants with lower vs. higher quartiles of 25(OH)D after 14 years, except for a small increase (4 mmHg) in systolic blood pressure when the lowest (<16 ng/mL) vs. the highest 25(OH)D quartiles (>25 ng/mL) were compared [39].
It is important to note that none of these studies evaluated the role of vitamin D repletion in the aforementioned outcomes. Observational studies cannot rule out other characteristics of these populations that may explain the associated cardiovascular effects. Well-designed randomized trials are needed to confirm or rule out the suspected associations [40].
Clinical Trials
Nonrandomized Open-Label Trials
A few small open-label studies have been conducted. One included 27 infants with vitamin D deficient rickets and ten controls. Both groups were administered one dose of 600,000 IU cholecalciferol and calcium for 2 weeks and showed an improvement in ejection fraction [41] Another study administered 50,000 units of cholecalciferol weekly for 12 weeks to 30 chronic hemodialysis patients with 25(OH)D levels <30 ng/mL followed by 20,000 IU weekly for another 12 weeks [37]. Interestingly, a significant reduction in left ventricular hypertrophy (by echocardiogram) and inflammatory markers was detected at 6 months. Similarly, several small short-term studies in hemodialysis patients with hyperparathyroidism experienced reductions in left ventricular hypertrophy after treatment with 1,25(OH)2D for 3 months [42, 43], or cholecalciferol for 1 year [44].
Randomized Trials
Randomized trials evaluating the effects of intervention with either 25(OH)D or active 1,25(OH)2D analogs on cardiovascular outcomes as a primary objective are very scarce (Table 11.2 ). Supported by extensive animal experiments and observational human studies suggesting an effect of vitamin D on left ventricular hypertrophy [10, 52–54], PRIMO (Paricalcitol Capsule Benefits in Renal Failure– Induced Cardiac Morbidity) was one of the first large randomized trials designed to evaluate the cardiac effects of vitamin D repletion. In PRIMO, 227 patients with Stage 3b-4 chronic kidney disease were randomized to receive either the active vitamin D compound paricalcitol or placebo for 48 weeks to evaluate its effect on left ventricular mass index (LVMI) [45]. Despite the supporting data in both animal and human studies, PRIMO found no effect of paricalcitol on LVMI (Fig. 11.1). All patients met left ventricular hypertrophy criteria by echocardiogram at baseline, but not by cardiac magnetic resonance, which may explain why this was a negative trial. A smaller study that included patients with severe LVH showed similar results [46]. A sub-analysis of PRIMO revealed significant reductions in left atrial volume index (Fig. 11.2), natriuretic peptides and cardiovascular hospitalizations (mostly due to heart failure) in the paricalcitol group [55]. Another very small study in children with severe heart failure and vitamin D deficiency randomized participants to either 12 weeks of cholecalciferol 1,000 IU or placebo and found a significant improvement in the heart failure score and left ventricular ejection fraction, among other parameters [47].
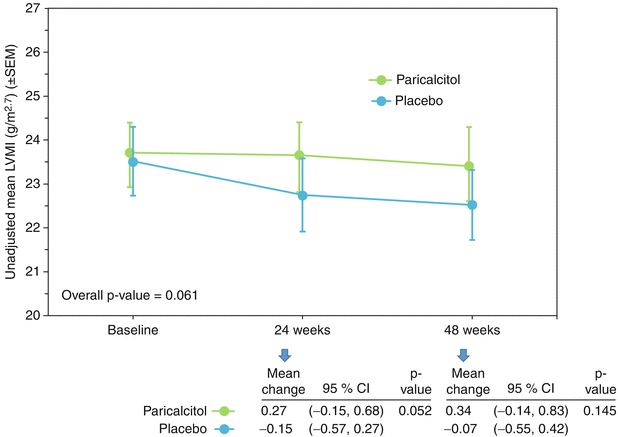
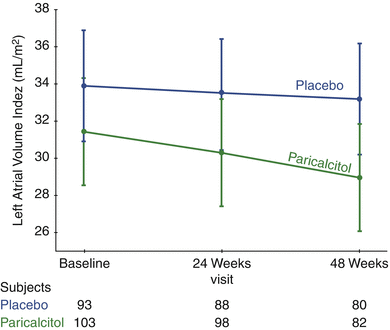
Table 11.2
Summary of randomized trials evaluating 25(OH)D or 1,25(OH)2D analogs on cardiovascular outcomes
Study | N | Population | Intervention | Follow-up | Outcome | Result |
|---|---|---|---|---|---|---|
Thadhani et al. [45] | 227 | CKD stage 3b-4 | Paricalcitol 2 μg/day or Placebo | 48 weeks | Change in LVMI | No effect in LVMI |
Wang et al. [46] | 60 | CKD stage 3–4 with left ventricular hypertrophy | Paricalcitol 1 μg/day or Placebo | 52 weeks | Change in LVMI | No effect in LVMI |
Shedeed et al. [47] | 80 | Infants with chronic heart failure | Cholecalciferol 1,000 IU/day or Placebo | 12 weeks | Left ventricular end-diastolic diameter | 32.8 ± 4.6 mm before and 24.9 ± 3.1 mm after treatment |
Larsen et al. [48] | 130 | Subjects with hypertension | Cholecalciferol 3,000 IU/day or Placebo | 20 weeks | 24 h blood pressure | BP significantly decreased in subjects with 25(OH)D <32 ng/ml |
Hsia et al. [49] | 36,282 | Postmenopausal women | Cholecalciferol 400 IU/day or Placebo | 7 years | Myocardial infarction, coronary death and stroke | No difference between groups |
Trivedi et al. [50] | 2,686 | Men and women over 65 years | Cholecalciferol 100,000 IU/4 months or Placebo | 5 years | Mortality by cardiovascular disease (fatal MI, stroke) | No difference between groups |
Prince et al. [51] | 302 | Older women with low 25(OH)D (<24 ng/mL) and previous history of falling | Ergocalciferol 1,000 IU/day or Placebo | 1 year | Ischemic heart disease (MI, angina) | No difference between groups |

Fig. 11.1
Unadjusted mean left ventricular mass index (LVMI) at baseline, week 24, and week 48 by treatment group

Fig. 11.2
Adjusted mean left atrial volume index (LAVi) at baseline, week 24, and week 48 by treatment group (Reprinted from Tamez et al. [55] with permission from Elsevier, Mosby, Inc; Copyright © 2012 Mosby, Inc. Terms and Conditions)
A handful of studies have evaluated the effect of cholecalciferol on blood pressure. A recent study in Denmark randomized 130 patients to 3,000 IU of cholecalciferol or placebo for 20 weeks. Although treatment with cholecalciferol was associated with a non-significant reduction in blood pressure compared to placebo, a post-hoc subset analysis restricted to vitamin D deficient patients (<32 ng/mL) revealed a small but statistically significant reduction in both systolic and diastolic blood pressure (4 and 3 mmHg respectively) [48]. Secondary analyses of other studies have yielded mixed results [56].
No studies have yet evaluated prevention of ischemic heart disease or cardiovascular mortality as the primary outcome. The Women’s Health Initiative randomized 36,282 postmenopausal women to calcium and vitamin D 200 IU twice daily or to placebo and found no significant difference in the rate of myocardial infarction, angina or stroke (secondary outcomes) after 7 years of follow up [49]. Similarly, another study of 2,686 participants (men and women) who were randomized to 100 000 IU of cholecalciferol or placebo administered every 4 months over 5 years found a non-significant improvement in all-cause mortality (mostly cardiovascular) [50]. Consistent with these findings, one smaller randomized Australian study that included 302 women with low 25(OH)D levels (<25 ng/mL) evaluated 1,000 IU daily of ergocalciferol or placebo and showed a non-significant reduction in cardiovascular events (2.0 % in ergocalciferol vs. 1.3 % placebo) [51].
The ongoing VITamin D and OmegA-3 TriaL (VITAL) aims to evaluate the role of cholecalciferol supplementation on cardiovascular diseases (primary outcome) [57]. VITAL has randomized 16,000 participants to either 2,000 IU of cholecalciferol daily or placebo with a median follow up of 5 years. Results of this well-designed trial are expected by 2016.
Biology
Cardiac tissue expresses the VDR gene, suggesting that vitamin D may have a direct effect on the heart [6, 7]. Furthermore, VDR expression is increased in hypertrophied hearts [58]. Cardiac tissue also expresses the 1-alpha hydroxylase and 24-hydroxylase genes, so in theory has the potential to convert 25(OH)D into active 1,25(OH)2D locally as well as convert 1,25(OH)2D into inactive 1,24,25-tetrahydroxy vitamin D [59].
Animal models that are vitamin D deficient develop cardiac hypertrophy, fibrosis and hypertension [60, 61]. Other animal models such as Dahl salt-sensitive rats, which develop hypertension and proteinuria, also become profoundly vitamin D deficient [52, 62]. These rats develop LVH, high left ventricular end diastolic pressures and elevated levels of natriuretic peptides that can be reversed with administration of 1,25(OH)2D analogs (such as paricalcitol) [52, 63] or doxercalciferol (a pro-hormone vitamin D2 analog) [63]. Similarly, vitamin D analogs prevent progression of LVH and heart failure [64] in animal models. One possible mechanism whereby hypertrophic changes occur is through a decrease in protein synthesis in cardiomyocytes, such as actin [54]. Treatment with paricalcitol has also been associated with higher VDR expression, reduction in Proliferating cell nuclear antigen (PCNA) [65], and inhibition of Protein kinase C alpha (PKCα) in the heart [51] Several other animal models including spontaneously hypertensive rats [66, 67], uremic rats [65], and even in swine [68] have shown similar associations. Among this abundance of positive data, one study utilizing a murine aortic constriction model failed to show that administration of a vitamin D analog could reverse cardiac hypertrophy, but reduced extracellular matrix proteins and natriuretic peptides [69]. Similarly, calcitriol reduced fibrosis and collagen synthesis in the hearts of rat models after partial nephrectomy, with no differences in heart weights [70].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


