Fig. 31.1
Amplatzer plug I (a). Amplatzer plug II (b). Amplatzer plug IV (c)
31.1.6.3 Techniques for Closing PAVM with Coils
To date, the most common approach to closing PAVM is embolization of the feeding artery using pushable fibered or detachable coils delivered via coaxial catheters.
Once a PAVM and its feeding arteries had been identified, selective catheterization of the target vessels is performed using a coaxial guide system with an outer 6-Fr 80 cm guide catheter and inner 5-Fr 100 cm end-hole coil delivery catheter (i.e., multipurpose catheter, Cook).
The added support provided by the guiding catheter prevents the inner coil delivery catheter from backing out of the target vessel during embolization, thereby allowing the coils to be delivered more precisely and in a tighter mass.
The use of such a coaxial guide system also allows smaller coils to be positioned within a larger anchor or scaffold coil.
Access to the middle and upper lobes can be challenging and is facilitated by the use of a 5-Fr Judkins left/right coronary catheter (cordis) or internal mammary catheter.
Once a feeding segmental artery is catheterized superselectively, the guiding catheter is advanced over the inner catheter to secure a stable position (i.e., placed in the parent segmental vessel), and the inner catheter is advanced into the vessel that feeds the malformation.
Hand-injected angiography(usually 4 frames/s) in multiple projections is performed to confirm position and define exact anatomy of the PAVM to determine site of implantation as well as size of the device to be used.
It is important to achieve as distal an embolization as possible in the feeding artery to avoid occluding branches to normal adjacent lung. This is especially true when multiple PAVMs are present and multiple indiscriminate proximal occlusions could result in a significant reduction in pulmonary blood supply.
Coil size is an important consideration. Undersized coils are at risk to pass through the malformation and becoming an embolic agent, while oversized coils may be difficult to form a tight nest. Many interventionalist empirically oversize the initial coil to the feeding vessel by at least 20 %. After placement of the first coil, additional coils must be positioned until blood flow to the PAVM has ceased. In order to create a dense, cross-sectional occlusion for durable result, packing of subsequent smaller coils in the center of the first deployed coil is essential (Fig. 31.2).
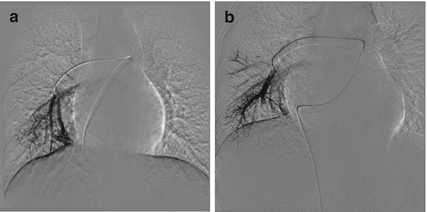

Fig. 31.2
(a) Pulmonary angiogram showing a PAVM of the right lower lobe. (b) Dense packing of two 6 mm coils producing complete occlusion
The “anchor or side-branch technique” and “scaffold technique” have been documented to be very useful in achieving complete cross-sectional occlusion and avoiding paradoxical embolization of the coil via the PAVM [1].
The “anchor technique” is characterized by the first 2 cm of the coil and is purposely anchored in a side branch close to the aneurysmal sac and the remainder of the coil positioned in the feeding artery and additional coils are densely packed so that cross-sectional occlusion is obtained. By securing the tip in a side branch, the risk of coil dislodgment is minimized.
The so-called scaffold technique is mainly used for high-flow vessels or when there is no anchoring vessel available. Initially, a high radial force, fibered coil with a diameter 2 mm larger than the feeding artery is placed to create a scaffold. Then several small diameter high radial force coils are placed as well into the endoskeleton, followed by several softer coils, until cross-sectional occlusion is obtained.
Packing of the aneurysm sac has been proposed as an alternative to feeding artery embolization when the feeding artery is too short to avoid sacrifice of large normal pulmonary artery branches or when the artery is a high-flow type with a higher risk of paradoxical embolization of coil.
Following the feeding artery that is catheterized superselectively with a 6-Fr guiding catheter, a coaxial microcatheter (i.e., a 2/2.6-Fr microcatheter (Excelsior; Boston Scientific)) is advanced coaxially through the catheter into the aneurysmal sac. Several microcoils (0.018 in.) are densely filled within the venous sac until a large matrix is established.
31.1.6.4 Techniques for Closing PAVM with Amplatzer Devices
For patients with PAVMs of large feeding artery or high-flow pattern, occlusion with coils is technically demanding and time consuming.
Alternatively, the recently developed Amplatzer vascular plug (AVP) appears to be an effective tool for embolization of PAVMs, particularly in patients with large outflow or short feeding arteries in whom embolization using coils entails a great risk of paradoxical embolization.
The AVP is made from densely woven nitinol mesh wires that can be delivered via small catheters such as standard 5–8-Fr coronary guiding catheters and can be repositioned multiple times prior to its final release.
Following pulmonary artery pressure recording and angiography, the feeding artery is selectively cannulated using an appropriate-sized guiding catheter (5-Fr guiding catheter for AVPs 4–8 mm in diameter, 6 Fr for AVPs 10–12 mm in diameter, and 8 Fr for AVPs 14–16 mm in diameter).
Once a suitable position has been achieved as distally as possible within the feeding vessel and beyond any branches to normal lung, the AVP is then delivered to the target area.
The diameter of the AVP is selected to be 30–50 % larger than the diameter of the feeding artery, according to the manufacturer’s recommendation. Satisfactory positioning of the AVP is confirmed by repeat arteriography via the guiding catheter before its final detachment. If suboptimally positioned, the AVP is resheathed and redeployed in a more appropriate site. Since the AVP does not cause instantaneous thrombosis and in high-flow situations thrombosis typically takes up to 15 min, control angiography should be performed for at least 15 min after deployment of the occluder.
The Amplatzer duct occluder (ADO) can also be an alternative for closing large PAVM (Fig. 31.3). After catheterization of the feeding vessel, a long delivery sheath is introduced over a stiff exchange wire and placed in the feeding artery as close to the malformation as possible. The size of the ADO selected for embolization should be 2–4 mm larger than the caliber of the feeding vessel at site of implantation.
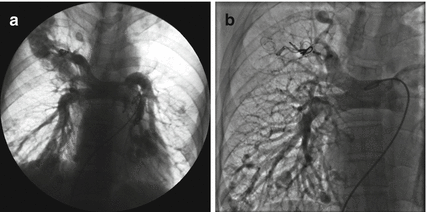

Fig. 31.3
A 29-year-old male with multiple PAVMs was referred for repeated PAVM occlusion. (a) Pulmonary angiogram revealed recanalization of previously embolized PAVMs. (b) PAVMs in the right upper and lower lobe were completely occluded with ADO
After embolization of the feeding artery or arteries by any of the above methods, repeated segmental and lobar angiography should be performed to assess for complete occlusion and any accessory feeding vessels that might also require embolization.
31.1.7 Expected Results
1.
Pulmonary angiogram post-embolization confirmed complete occlusion of the PAVMs.
2.
A significant improvement in systemic arterial oxygen saturation and sustained relief of clinical symptoms attributed to the PAVMs on follow-up are obtained after embolization.
3.
Contrast-enhanced CT at follow-up showed that the PAVMs remained occluded, with a significant shrinkage of the vein sac or complete resolution of the malformations.
31.1.8 Complications and Its Management
To date, there is no mortality occurred during the procedure and long-term follow-up. The complications of transcatheter embolization of PAVMs documented in the literature have been infrequent and are listed as follows:
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


