5 Ventricular Septal Defect
I. CASE
A. Fetal echocardiography findings
1. Fetal echocardiography reveals situs solitus of the atria, levocardia, left aortic arch, and normal cardiothoracic ratio (0.3).
2. The four-chamber view is normal.
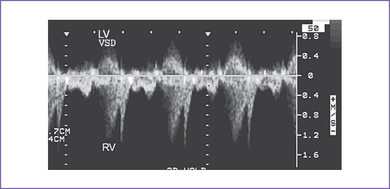
3. There is a moderate (5 mm) perimembranous ventricular septal defect (VSD) (subaortic), with bidirectional shunting and velocity of 1 m/s (Fig. 5-1).
4. Outflow assessment shows normally related great arteries with normal valve morphology and size. The pulmonary artery is anterior to and to the left of the aorta.
5. Both the aortic arch and ductal arch are of equal size and have normal antegrade flow.
6. There is a nonrestricted right-to-left shunt across the foramen ovale; however, no aneurysm or atrial septal tissue could be seen across the fossa ovalis.
7. The pulmonary venous drainage is normal.
8. The RV and left Tei indices (myocardial performance index) were normal. There are no signs of heart failure.
B. Cardiovascular profile score
The cardiovascular profile score is 10/10. The arterial and venous flow patterns are normal.
D. Fetal management and counseling
a. Because of the possibility of aneuploidy, amniocentesis was offered but was declined.
b. Abnormal karyotype with a single VSD is common in a fetus with a trisomy and otherwise structurally normal heart.
a. Serial antenatal fetal echocardiography studies are performed at 6- to 8-week intervals to assess the size of the defect and compare it with the aortic root size serially.
b. At each visit, a renewed search is made for possible developing associated cardiac lesions, such as coarctation of the aorta.
c. In an outlet defect, pulmonary stenosis can appear later in pregnancy, as evidenced by a pulmonary artery smaller than the aorta, thus evolving into tetralogy of Fallot or pulmonary valve stenosis.
d. The size of the ascending aorta and transverse aortic arch is monitored.
e. Size and function of both ventricles are monitored. The size of the ventricles should be compared.
f. The size of the aortic valve annulus is monitored; check for subaortic stenosis.
g. The size and direction of flow across the foramen ovale should be checked to ensure the right-to-left flow is normal in the fetus.
h. The patency of the ductus arteriosus must be ensured.
i. Development of heart failure would not be expected in this condition.
j. Patient management options must be re-evaluated in view of any evolving lesions or new documentation of extracardiac findings that might alter the outcome.
E. Delivery
1. If the fetus remains well compensated and the lesion is isolated, delivery can be normal at term in a secondary care center.
2. Once the baby is delivered, an echocardiogram should be performed to determine the size, number, and hemodynamics of the VSD(s) and to exclude additional pathology. This could be done within the first 2 weeks of delivery if the third-trimester fetal echocardiogram suggests no critical heart disease.
F. Neonatal management
With normal appearance and no other malformations, the baby should go home with the mother.
a. Heart failure with a VSD often does not manifest for several weeks after birth.
b. If the size of the arch is questionable, the baby should be managed as a neonate with possible coarctation (see Chapter 14).
c. Antifailure medication in the form of oral digoxin and furosemide can be started in the hospital, if surgery is likely, or at the first sign of congestive heart failure (see Chapter 30). Use of angiotensin-converting enzyme (ACE) inhibition to reduce the systemic vascular resistance out of proportion to the pulmonary vascular resistance can also assist in managing the heart failure by reducing the net shunting or  p/
p/ s (ratio of pulmonary flow to systemic flow).
s (ratio of pulmonary flow to systemic flow).
d. If the VSD is large and there is evidence of significant shunting, a high-calorie formula may be needed to maintain growth, given the high metabolic rate such infants can have.
a. The majority of VSDs (>50%) close spontaneously, usually in the first year of life. Only a small percentage of VSDs require surgery.
b. Surgery is confined to patients with elevation of pulmonary artery pressure, significant heart failure, failure to thrive, and evolution of additional pathology such as subaortic obstruction, aortic valve prolapse with regurgitation, and right ventricular outflow tract (RVOT) obstruction.
c. The surgical procedure of choice is primary open heart patch closure of the VSD and atrial septal defect (ASD) through the right atrium (RA) on bypass. Most patients are hospitalized 3 to 10 days after surgery for VSD and ASD closure
d. The risks of surgery are low: 1% to 2% for mortality or a complication such as complete heart block.
e. Contraindications for surgery after 1 year of age.
G. Follow-up
1. Subacute bacterial endocarditis (SBE) prophylaxis should be continued as long as any VSD is present and for 6 months after successful closure of the defect. ASD is not an indication for SBE prophylaxis.
2. Yearly visits to the cardiologist are indicated to detect late problems with arrhythmia such as sinus bradycardia from sinus node dysfunction or atrial tachycardia. A new heart murmur could indicate development of subaortic stenosis.
I. Outcome of this case
1. The baby was delivered at term weighing 3.0 kg. Apgar scores were good: 8 and 9 at 1 and 5 minutes, respectively. Pulse oximeter reading was 98% in room air.
2. Echocardiogram confirmed the diagnosis of a large perimembranous VSD (Fig. 5-2), a small apical muscular VSD (Fig. 5-3), and a moderate-sized secundum ASD.
3. Medical therapy with digoxin, furosemide, and captopril was given for the first 8 weeks after birth.
4. Due to poor weight gain, the baby was referred to a dietician.
5. Surgery was performed at 2 months of age with division of the patent ductus arteriosus, patch closure of the secundum ASD, and patch closure of the VSD. Postoperatively, a large pericardial effusion developed that required pericardiocentesis.
6. The patient was discharged on PO digoxin and furosemide at 2 weeks after surgery.
7. At the 2-week follow-up visit in the cardiologist’s office, the baby was well and medications were stopped. SBE prophylaxis was continued for 6 months and then discontinued. He was seen yearly thereafter.
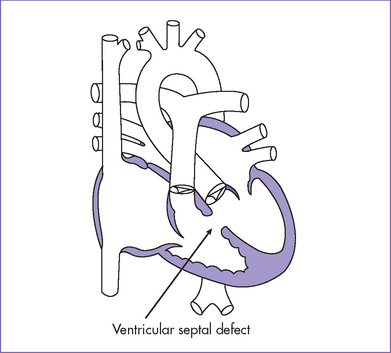
Fig. 5-2 Perimembranous ventricular septal defect (arrow).
(Modified from Mullins CE, Mayer DC: Congenital Heart Disease: A Diagrammatic Atlas. New York, Liss, 1988.)
II. YOUR HANDY REFERENCE
A. Ventricular septal defect
a. VSDs are the commonest form of congenital heart disease detected in infancy, accounting for more than 30% of the total cases of congenital heart disease. The incidence is 2 in 1000 live births.
b. The spectrum of VSDs seen in prenatal life is very different from that manifesting postnatally. Isolated VSDs constituted only 6% of a large series of congenital heart diseases identified prenatally.
c. Perimembranous and small VSDs (the most common type postnatally) are rarely seen prenatally, particularly in isolation, and the moderate and large defects predominate.
a. VSDs are the most common type of congenital heart malformation to be overlooked in the fetus. Their detection prenatally depends on the image quality, the size and site of the defect, and the color Doppler capabilities of the ultrasound machine. A large VSD may be seen only on imaging.
b. Most overlooked defects tend to be small.
c. VSDs are commonly associated with other cardiac defects as part of more complex disease.
d. VSDs can vary in location and are divided into inlet, outlet, perimembranous, and muscular defects.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree