Ventricular Septal Defect
Christopher H. May
I. Introduction
A. Ventricular septal defect (VSD) is one of the most common congenital heart defects in both children and adults. The prevalence in neonates has been reported to be as high as 5% when screened with color Doppler echocardiography, although most of these are miniscule defects that close spontaneously within the first year. Thus the true prevalence is difficult to ascertain, given that many defects close spontaneously
and patients are frequently asymptomatic with smaller lesions. VSDs are frequently associated with other congenital defects, particularly infundibular stenosis or valvar pulmonary stenosis. Isolated VSDs account for about 20% to 25% of all congenital heart defects in childhood. Unlike many other congenital abnormalities, males and females appear to be affected equally.
and patients are frequently asymptomatic with smaller lesions. VSDs are frequently associated with other congenital defects, particularly infundibular stenosis or valvar pulmonary stenosis. Isolated VSDs account for about 20% to 25% of all congenital heart defects in childhood. Unlike many other congenital abnormalities, males and females appear to be affected equally.
B. Isolated VSDs are found in approximately 10% of adult patients with congenital heart disease. This reflects the natural tendency for spontaneous closure during infancy and an improved ability to confirm the diagnosis in childhood, which leads to surgical closure.
C. Natural history
1. Spontaneous closure occurs most commonly with smaller, restrictive VSDs, usually before the age of 2 years. In general, nearly 35% of perimembranous defects close spontaneously and 75% to 80% of all small VSDs close spontaneously by 10 years of age. These higher rates of spontaneous closure in more recent series are a reflection of the ability to diagnose much smaller defects with more contemporary echocardiographic modalities. Large and nonrestrictive defects have significantly lower spontaneous closure rates (approximately 10% to 15%); malalignment defects rarely close spontaneously. Defects close by two mechanisms: (1) by muscular septum growth and (2) by “aneurysmal tissue” from a septal leaflet of the tricuspid valve as in the case of perimembranous defects. For VSDs that persist, a restrictive nature can protect the patient from pulmonary vascular injury given the flow-limiting nature of these defects.
2. Endocarditis is a risk because of the presence of a high-velocity, turbulent jet into the right ventricle. Endocarditis most frequently involves the septal leaflet of the tricuspid valve apparatus at the point of jet impact. The risk of endocarditis is roughly 4% to 10% for the first 30 years of life. Muscular VSDs have a lower incidence of endocarditis, as the jet is attenuated prior to reaching the tricuspid valve.
3. A large VSD during childhood is typically associated with significant left-toright shunt and eventual development of congestive heart failure. Children with very large defects usually present during infancy or early childhood with signs and symptoms of heart failure and pulmonary hypertension. Patients with moderate-sized VSDs can survive to adulthood before detection. Given the gradual development of symptoms in these patients, they may not present until late in the disease course. In these patients, the excess right-sided flow may lead to pulmonary vascular disease and Eisenmenger physiology if left untreated. As pulmonary vascular resistance increases, the left-to-right shunt changes to a rightto-left flow. The VSD murmur disappears during this transition and is often replaced by the murmur of tricuspid regurgitation. After Eisenmenger physiology has developed, patients rarely survive beyond the fourth decade. Complications in patients with Eisenmenger syndrome include pulmonary hemorrhage, endocarditis, cerebral abscess (from hypoxemia), ventricular arrhythmias, and the complications associated with erythrocytosis. Poor prognostic factors in this population include syncope, congestive failure, and hemoptysis.
4. Risk factors for decreased survival include cardiomegaly seen on the chest radiograph; elevated pulmonary artery systolic pressure (> 60 mm Hg and/or more than one-half of the systemic pressure); cardiovascular symptoms such as shortness of breath, fatigue, or dyspnea on exertion; and progressive aortic insufficiency. Good prognostic factors include normal left ventricular (LV) size and function, small left-to-right shunt, normal pulmonary pressures or resistance, an intact vasodilator response in the pulmonary vasculature, and a lack of symptoms.
5. Genetic factors play a significant role in this disease, as in other forms of congenital heart disease. Having an affected father increases the risk of VSD in
the offspring to 2%; moreover, an affected mother appears to confer an even higher risk of recurrence in offspring—as high as 6% to 10%. In general, VSDs arise due to a combination of polygenic, multifactorial abnormalities. However, several monogenetic abnormalities leading to VSDs such as mutations in the transcription factors TBX5 and GATA4 have recently been described.
the offspring to 2%; moreover, an affected mother appears to confer an even higher risk of recurrence in offspring—as high as 6% to 10%. In general, VSDs arise due to a combination of polygenic, multifactorial abnormalities. However, several monogenetic abnormalities leading to VSDs such as mutations in the transcription factors TBX5 and GATA4 have recently been described.
II. ANATOMY
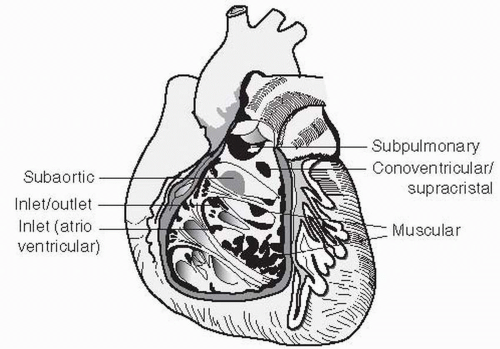
A. Embryology. Partitioning of the ventricular mass begins as a muscular ridge in the floor of the ventricle near the apex. This ridge later undergoes active growth, which forms the muscular ventricular septum. Concomitantly, the endocardial cushions fuse and the two regions meet, completing closure of the interventricular foramen. Figure 30.1 shows anatomic localization of VSDs.
B. Defect size. The consequences of a VSD depend on the size of the defect and the pulmonary and systemic vascular resistances. Smaller defects provide higher resistance to flow and will have little impact on right-sided flow. The VSD is described as small when the defect size is less than one-third of the size of the aortic root, moderate when the defect size is less than one-half of the size of the aortic root, and large when the defect size is equal to or larger than the size of the aortic root. However, other indirect measures, including clinical signs and symptoms and echocardiographic features, must be taken into consideration when determining the size and clinical significance of a VSD. VSD size is often classified on the basis of its hemodynamic consequences:
1. Restrictive VSDs result in a significant pressure gradient between the left and right ventricles (e.g., pulmonary/aortic systolic pressure ratio < 0.3) and are associated with a small shunt (Qp/Qs ≤ 1.4:1).
2. Moderately restrictive VSDs produce an intermediate interventricular gradient and result in a moderate shunt (Qp/Qs = 1.4 to 2.2:1).
3. Nonrestrictive VSDs are usually larger than 1 cm2 and are associated with a large shunt (Qp/Qs> 2.2:1). The pressures in the left ventricle and right ventricle will eventually approach equalization, and the amount of flow across the defect will be determined by the ratio of pulmonary-to-systemic vascular resistance.
C. VSD types
1. Membranous defects are the most common type, accounting for approximately 70% to 80% of VSDs. The membranous septum is the area under the aortic valve on the left side and next to the septal leaflet of the tricuspid valve on the right side. Most of these defects extend into the infundibular region and are then referred to as perimembranous. Membranous defects are less likely to be associated with additional intracardiac defects and have a high rate of spontaneous closure. However, when there is malalignment of the defect, spontaneous closure is unlikely.
2. Muscular defects account for approximately 5% to 20% of VSDs and can be single or multiple (i.e., Swiss cheese septum). These defects, when single, also have a high spontaneous closure rate.
3. Inlet or atrioventricular (AV) canal—type defects account for approximately 5% to 8% of cases. These defects rarely close spontaneously, are usually large, and are associated with abnormalities of the AV valves. These abnormalities range from cleft mitral and tricuspid valves to the common AV valve, as seen in complete AV canal defect. This type of defect in the inlet ventricular septum is commonly seen in patients with Down syndrome (Trisomy 21).
4. Supracristal or subaortic defects account for approximately 5% to 7% of cases and are located immediately beneath the pulmonary and aortic valves. These defects vary in size but are often small. Because of their proximity to the aortic valve, aortic leaflet tissue can invaginate and result in their closure, with the unfortunate result of significant aortic regurgitation.
D. Associated lesions. Approximately 20% of VSDs are associated with many other forms of congenital heart disease, including aortic coarctation, bicuspid aortic valve, and patent ductus arteriosus. Of patients who present with a VSD, 5% to 10% will develop aortic regurgitation because of poor support of the right coronary cusp and the Venturi effect caused by the VSD jet, resulting in prolapse of one of the aortic valve leaflets. Discrete, fibrous subaortic stenosis and right ventricular (RV) outflow tract obstruction are less common associations. Less than 10% develop subvalvular pulmonary stenosis or an obstructive muscle bundle referred to as a double-chamber right ventricle. VSD is also associated with transposition of the great arteries, tetralogy of Fallot, and Trisomies 13, 18, and 21.
III. CLINICAL PRESENTATION.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree