Fig. 8.1
An artist rendition of the polyester implant, a concept of the Acorn cardiac support device
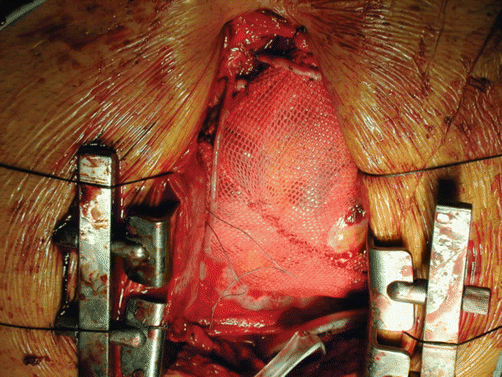
Fig. 8.2
Operative photograph of one of the first implants of the Acorn cardiac support device. Operative photograph of a contained ventricle in the setting of CABG (1999). Note the window in the mesh cut away for a distal anastomosis of a graft
Salient findings in the Acorn Trial in the US were identified. For instance, among patients with heart failure and dilated CMP, the CorCap cardiac support device was associated with a significantly improved primary composite endpoint viz. Clinical status based on rates of death, major cardiac procedures indicative of HF progression, or changes in NYHA class.
The primary endpoint has been largely driven by significant reduction in major cardiac procedures and a trend toward improvement in NYHA in the CSD group. No difference between the two groups in mortality.
The CSD group had significantly lower left ventricular ESV/EDV, greater enhancement in sphericity index, noteworthy improvements in measures of quality-of-life than the control group. However, the groups had similar LV ejection fractions and comparable rates of adverse events, including repeat hospitalizations.
The Acorn device was deliberated by the FDA panel three times. The panel voted overwhelmingly on all three occasions to deny approval for human use. Acorn eventually asked for a further review through the FDA ombudsman. Finally, a recommendation was made that asked for another 50 patient study to be performed. The Acorn CSD was then re-engineered to be implanted in a minimally invasive fashion through a left mini-thoracotomy. This was after many iterations of the implant tool were developed at the Texas Heart Institute by Dr. William “Billy” Cohn. Unfortunately, the company ran out of money and is now defunct.
Interestingly, a 2012 publication from Journal of Thoracic and Cardiovascular Surgery, Mann et al. probed the 5-year results of the CorCap and the non-MR stratum. Conclusively, the CSD demonstrated no long-term adverse effects on mortality, concurrently illustrating a reduction in the LV remodeling. Moreover, results of a study involving the implant of the CorCap device utilizing minimal invasive conditions are pending [9].
In order to make this approach worthy of clinical application, the device has to be dramatically modified to allow subsequent interventions safe. In addition it would be preferable to use smart materials that are actively promote reverse remodeling of the ventricles.
Unfortunately, drawbacks were identified and the trial, though painted as positive in media releases, was turned down by the FDA panel multiple times. Their reasons were many fold and make thought-provoking reading:
Lack of complete follow up
Data not validated by a core lab
Composite end point was significant, but no single factor achieved significance of its own
No change in left ventricular ejection fraction between the groups
No differences in heart failure admissions or mortality between the two groups.
The need for clear and definite end points to be delineated before the study was started.
The decreased need for new cardiac procedures in the study group was perceived as reluctance on the part of implanting surgeons to re-operate on a patient with an increased risk of severe adhesions.
Another motivating lesson was the Hawthorne effect within each group that displayed continued care and repeated follow-up actually benefited patients and reduced heart failure symptoms and subsequently admissions.
Long-term follow-up of patients undergoing mitral valve repair along with containment with the Acorn Cardiac Support Device demonstrated some benefit at 5 years (Acker et al.).
Nevertheless, the areas that showed significant difference were those concerned with left ventricular dimensions and size, with the study group validating that the ventricles were contained at the smaller size. Our own work in animals illustrated that the best results were obtained when sheep were contained in a moderate phase of heart failure (akin to class II heart failure) [10]. Containment late in the cycle of dilatation and heart failure provided marginal benefit with improved survival in sheep with contained ventricles [11].
At a molecular and cellular level, a 2003 study in dogs showed long-term benefit of CSD in the attenuation of the LV remodeling process by investigating various gene expression molecules, cardiac myocytes, and stretch response proteins [8].
Alteration of Left Ventricular Wall Stress by Shape Change
Myocor Approach
Myocor was a company that founded in 1996 to utilize the theory of ventricular shape alteration to reduce wall stress (Fig. 8.3). They pre-supposed the left ventricle to be a cylinder or a sphere. If Laplace’s law were applied, wall stress increases proportionate to the radius of curvature. To alter and diminish this, Myocor proposed skewering the heart with a tensioning chord or splint that would change the left ventricle in cross section from a large circle to two small circles or a bi-lobed ventricle (Fig. 8.3). Preliminary studies with the Myosplint in human patients were performed in Munich with poor results. McCarthy implanted a few of the Myosplint devices acutely in patients undergoing transplantation prior to explant of the heart with equally poor results. Myocor had mixed clinical success with the CoApsys device which utilized a portion of these elements, but this worked predominantly to correct functional MR. The Myocor experience in correcting functional mitral regurgitation was promising. Myocor was not able to express clinical significance of any of their approaches during the operation of the trial and depleted of funds. This company has been defunct for over 4 years now.

Fig. 8.3
The concept behind the Myocor Myosplint is by altering the short axis of the left ventricle into two bilobed spheres, thereby reducing wall stress
Cardioclasp
In keeping with the shape change and altering the stresses on the myocardium, Dr. David Melvin, from Cincinnati developed another device that looked and behaved like a vice around the ventricle (Fig. 8.4). Although conceptually interesting, the animal studies led only to mildly promising results, halting progression to clinical evaluation.
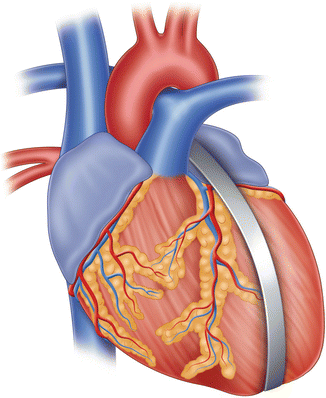

Fig. 8.4
Illustration of the Cardioclasp device. The anterior and posterior bars are aligned to the long axis of the left ventricle, adjacent to the left anterior descending and posterior descending coronary arteries. While this concept is interesting, the animal studies showed only mild promise and this concept did not progress to clinical evaluation [21]
Dynamic Ventricular Restraint(by Corset)
The concept of dynamic ventricular restraint was developed by a group of researchers at the Cedars Sinai Medical Center. The notion was tested in animals in Melbourne, Australia by Raman and colleagues with a prototype restraint device that had inflatable balloon panels along its length, linked to a portacath type device. The plan was to gradually infuse saline into the portacath so as to slowly increase the constraint over time. Unfortunately, the tubing connecting to the balloons often leaked and there was no indication that the balloons actually inflated enough to cause significant reduction in ventricular size. This device did not proceed beyond initial proof of concept studies in animals. This is quite relevant to the development of other devices by various startup groups that have touted the benefits of inflatable balloon panels within the pericardium.
Quantitative Ventricular Restraint (QVR)
Brigham and Women’s developed a quantitative, adjustable ventricular restraint device that is a double layered, semi-elliptical, polyurethane balloon connected to a portacath line (Fig. 8.5). The QVR balloon is placed below the atria, around both ventricles, and secured to the heart along the arteriovenous groove. The portacath is tunneled through the left anterior chest wall. Contrast to prior static devices, measurable restraint levels can be quantified and experimented in the ovine model, distinguishing reverse remodeling at a given extrinsic balloon pressure. Additionally, short-term changes were declared as primary endpoints including decreased transmural pressures (Ptm) and oxygenation consumption of the myocardium. Moreover, they noticed that a decrease in device pressure from lessened Ptm slowed the reverse remodeling process. Limitations to this study were acknowledged as small sample size and long-term effects, suggesting the aid of cardiac imaging, including magnetic resonance [12].
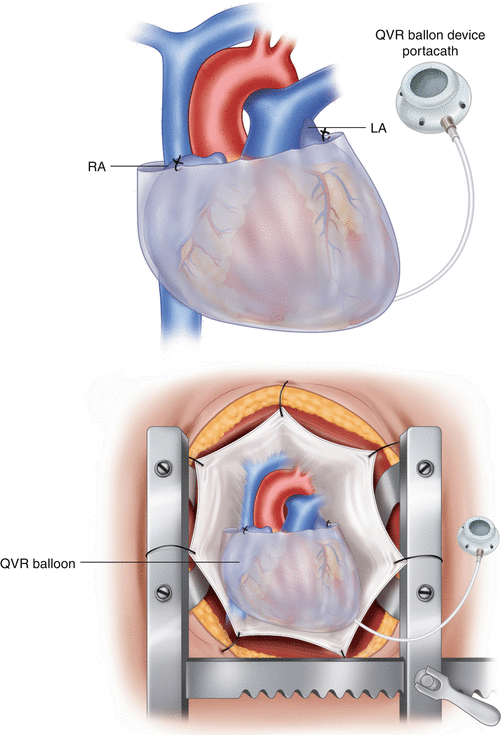

Fig. 8.5
(a, b) Photographs of the semi-ellipsoidal QVR Balloon Device with its portacath (Modified from Ghanta et al. [12])
Paracor
The concept of dynamic ventricular restraint has been spoken about and developed initially by another startup company called Paracor (Figs. 8.6 and 8.7). This device, the HeartNet, utilizes a meshwork of nitinol coated with silicone that can be laid around the ventricles to ensure dynamic restraint of the dilating ventricles. There is very little published data about the efficacy of this approach in experimental animals. The first human implant was performed at the Ohio State University on May 19, 2005. The clinical results of the pivotal study were equivocal and this halted the study. As with prior passive ventricular devices, heart failure symptoms in conjunction with measurements in chamber physiology revealed noticeable improvements including dimensions, end-diastolic and end-systolic volumes. Further analyses showed that there might be some benefit in patients who also had bi-ventricular pacing therapy.
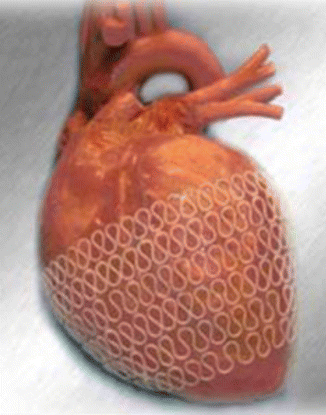
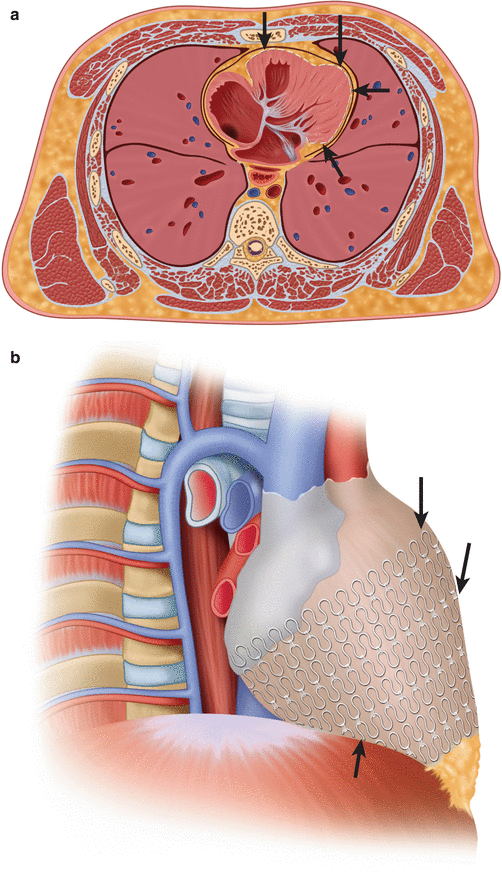

Fig. 8.6
The Paracor device, which is a nitinol mesh coated with polyurethane; this is slipped over the heart preventing progressive dilatation

Fig. 8.7
(a, b) Axial and Coronal CT images of a Paracor device in a 61-year-old male with heart failure. Further analyses showed that there might be some benefit in patients who also had bi-ventricular pacing therapy (Modified from [22])
Infarct Restraint and/or Constraint
Extensive studies have been performed by the Edmunds’ laboratory at the University of Pennsylvania focusing on localized constraint or restraint upon dyskinetic or akinetic areas of the ventricles. They revealed that a certain type of a non-absorbable material that is applied and secured to the epicardial surface of the ventricle in an area of akinetic or dyskinetic scar helps reduce infarct expansion [13].
Our own adoption of this principle has been in the use of Teflon strips in reinforcing the scar over a reconstructed ventricle in the setting of a modified Dor procedure or a Left ventricular reconstructive procedure.
Devices in the Minimally Invasive Treatment of Mitral Regurgitation
Percutaneous Mitral Valve Approaches
Several minimally invasive percutaneous devices have developed utilizing the coronary sinus as the fulcrum for tensioning rods to correct mitral regurgitation. Several companies have introduced these devices through both animal and phase I/II human trials.
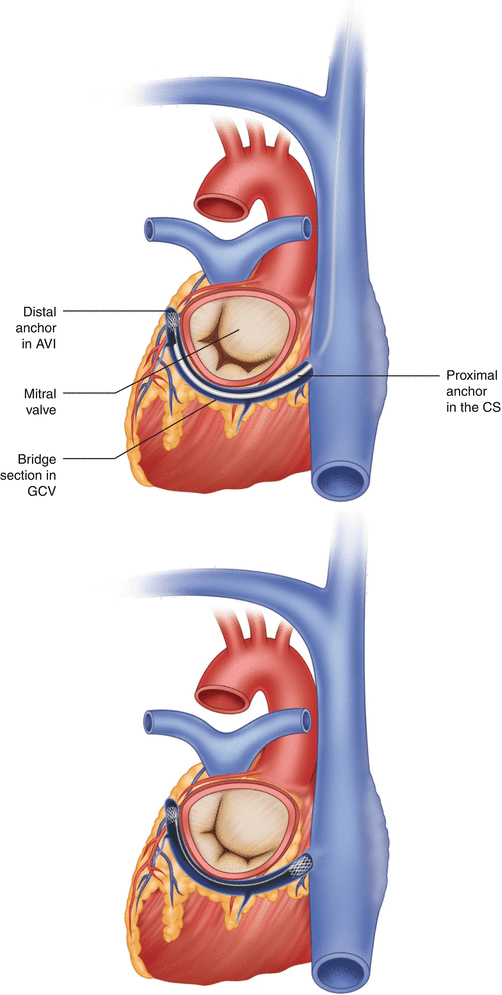
Edwards Monarch Device
Edwards Lifesciences (Irvine, CA) developed a percutaneous coronary sinus (CS) annuloplasty device (Fig. 8.8) in which most recently was followed with a publication of the 1-year results (EVOLUTION trial). A phase one human trial, largest of its kind to date, has shown improvement in mitral regurgitation in 50 % of its sample size at the 6-month mark, an 86 % reduction in heart failure symptomatology. There were no in-hospital deaths.