Ventricular Arrhythmias and Bundle-Branch Block
Overview
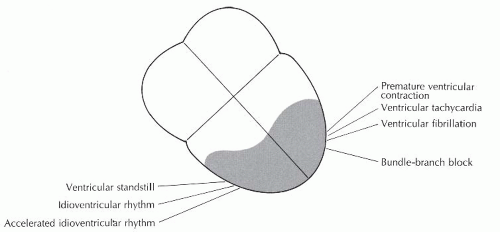
The three preceding chapters have focused on supraventricular arrhythmias. Supraventricular arrhythmias refer to those rhythms that originate above the bundle branches and include the sinus, atrial, and junctional rhythms. The electrical impulse produced by supraventricular rhythms follows the normal conduction pathway, resulting in simultaneous depolarization of the right and left ventricles. The resulting QRS complex is narrow (0.10 second or less in duration). Ventricular beats and rhythms (Figure 9-1) originate below the bundle of His in a pacemaker site in either the right or left ventricle. When impulses arise in the ventricles, the impulse does not enter the normal conduction pathway, but travels from cell to cell through the myocardium, depolarizing the ventricles asynchronously. Therefore, the ventricles are not stimulated simultaneously and the stimulus spreads through the ventricles in an aberrant manner, resulting in a wide QRS complex of 0.12 second or greater.
Since ventricular depolarization is abnormal, ventricular repolarization will also be abnormal, resulting in changes in the ST segments and T waves. The ST segments and T waves will slope in the opposite direction from the main QRS deflection (if the ectopic QRS complex is predominantly negative, the ST segment is usually elevated and the T wave positive; if the ectopic QRS complex is predominantly positive, the ST segment is usually depressed and the T wave negative). A P wave is not produced in ventricular rhythms.
Ventricular arrhythmias include premature ventricular contractions (PVCs), ventricular tachycardia (VT), ventricular fibrillation (VF), idioventricular rhythm, accelerated idioventricular rhythm, and ventricular standstill. All of these rhythms are associated with a wide QRS complex (except VF and ventricular standstill, which do not have QRS complexes). Because the ventricles are the least efficient of the heart’s pacemakers, most of these rhythms are (or have the potential to be) life-threatening and demand prompt recognition and treatment.
The electrical impulse in bundle-branch block originates in the sinus node, not in ventricular tissue, but a discussion of bundle-branch block is included in this rhythm group because of the location of the block within the ventricles and the wide QRS complex.
Bundle-branch block
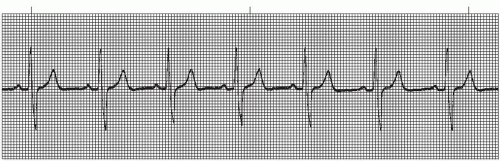
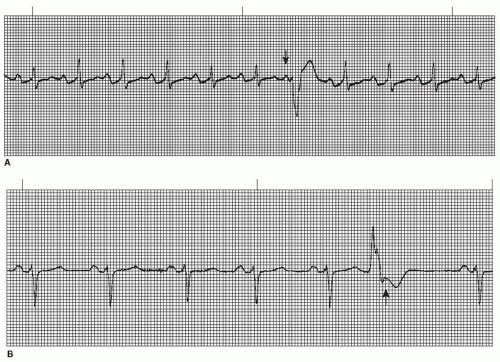
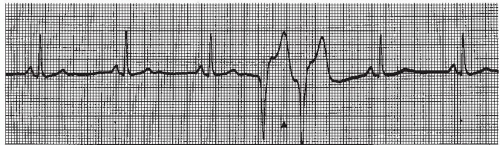
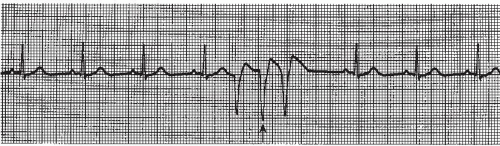
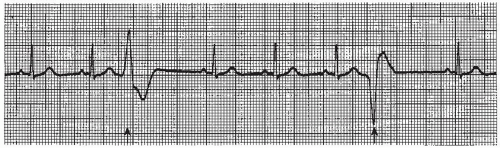
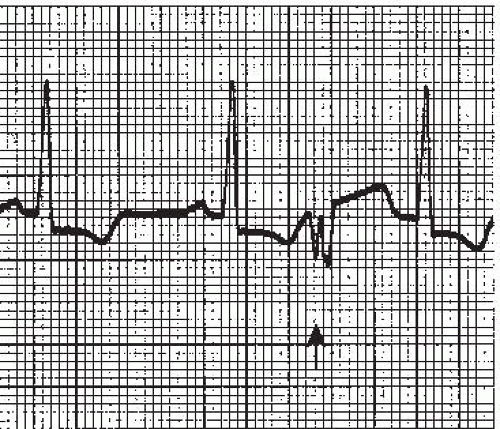
The intraventricular conduction system consists of the right bundle branch and the left main bundle branch, which divides into two fascicles: an anterior fascicle and a posterior fascicle. Block may occur in any part of this conduction system. Normally, the electrical impulses travel through the right bundle branch and the left bundle branch and its fascicles at the same time, causing simultaneous depolarization of the right and left ventricles, resulting in normal depolarization and a QRS duration of 0.10 second or less. When one of the bundle branches is blocked, the electrical impulse travels down the intact bundle, depolarizing that ventricle first, then the impulse progresses through the interventricular septum to depolarize the other ventricle. Depolarization of one ventricle before the other is called sequential depolarization. Depolarization of the ventricles is delayed, resulting in a wide QRS complex of 0.12 second or greater. The presence of a bundle-branch block (Figures 9-2, 9-3 and 9-4 and Box 9-1) can be recognized by a monitoring lead. Differentiating between right and left bundle-branch block requires a 12-lead electrocardiogram (ECG).
Box 9-1. Bundle-branch block: Identifying ECG features
Rhythm: Regular
Rate: That of the underlying rhythm (usually sinus)
P waves: Sinus
PR interval: Normal (0.12 to 0.20 second)
QRS complex: Wide (0.12 second or greater)
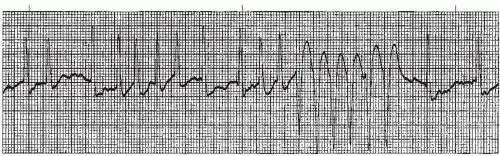
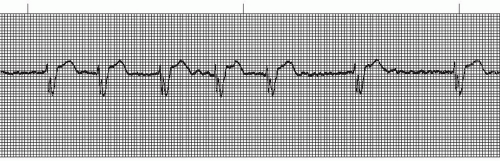 Figure 9.4. Atrial fibrillation with bundle-branch block. Rhythm: Irregular Rate: 70 beats/minute P waves: Fibrillatory waves present PR interval: Not measurable QRS complex: 0.14 to 0.16 second. |
Right bundle-branch block (RBBB) may be present in healthy individuals with no apparent underlying heart disease, but more commonly occurs in the presence of coronary artery disease (the most common cause). RBBB may be temporary or chronic. Occasionally, RBBB may appear only when the heart rate exceeds a certain critical level (rate-related BBB). Common causes include anteroseptal myocardial infarction (MI), pulmonary embolism, congestive heart failure, pericarditis, hypertensive heart disease, cardiomyopathy, congenital RBBB, and degenerative disease of the electrical conduction system.
Left bundle-branch block (LBBB) is rarely seen in individuals with healthy hearts. It appears most commonly in elderly individuals with diseased hearts. LBBB may be temporary or chronic, and may be rate-related. The most common cause is hypertensive heart disease. Other causes are the same as with RBBB.
Specific treatment is usually not indicated for a bundle-branch block. Cardiac pacing may be indicated if the bundle-branch block develops as a result of acute MI or in the presence of AV block.
Premature ventricular contractions
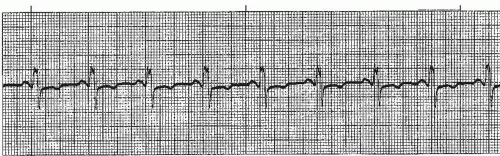
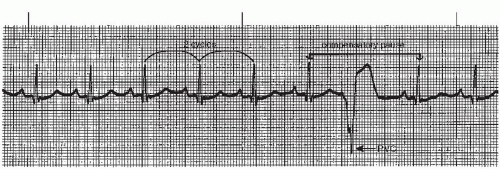
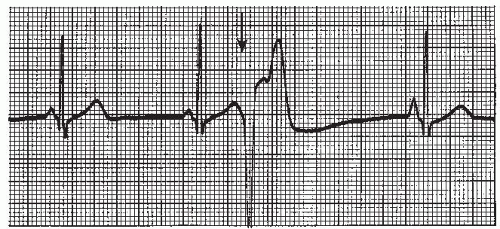
A premature ventricular contraction (PVC) (Figures 9-5, 9-6, 9-7, 9-8, 9-9, 9-10, 9-11, 9-12, 9-13 and 9-14 and Box 9-2) is a premature, ectopic impulse that arises below the bundle of His in the ventricles. PVCs occur as a result of reentry in the ventricles, enhanced automaticity of a focus in the ventricles, or triggered activity occurring during ventricular repolarization. PVCs have the following characteristics:
▪ The QRS is premature.
▪ A P wave isn’t associated with the PVC. Normally the P wave of the underlying rhythm (usually sinus) is obscured within the PVC, but sometimes it appears just before or after the PVC in the ST segment or T wave (see Figure 9-6).
▪ The QRS is wide (0.12 second or greater) and the morphology is different from the QRS complexes of the underlying rhythm.
▪ The ST segment and T wave slope in the opposite direction from the main QRS deflection (if the ectopic QRS complex is predominantly negative, the ST segment is usually elevated and the T waves positive; if the ectopic QRS complex is predominantly positive, the ST segment is usually depressed and the T wave negative).
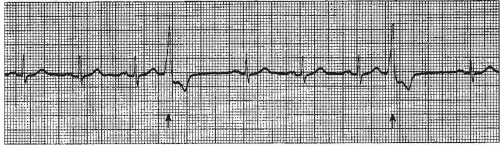
▪ The pause associated with the PVC is usually compensatory (the measurement from the beat before the PVC to the beat after the PVC is equal to two R-R intervals of the underlying rhythm, Figure 9-5). The underlying rhythm must be regular to determine a compensatory pause.
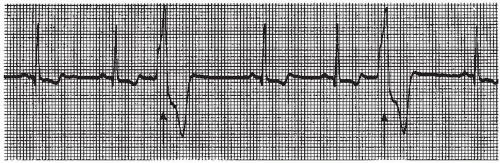
PVCs may occur in various patterns. They may appear as a single beat (Figure 9-5), every other beat (bigeminal pattern, Figure 9-7), every third beat (trigeminal pattern, Figure 9-8), every fourth beat (quadrigeminal pattern, Figure 9-9), in pairs (also called couplets, Figure 9-10), or in runs (Figure 9-11). A run of three or more consecutive PVCs constitutes a rhythm. The rate will determine which rhythm is present (idioventricular rhythm, accelerated idioventricular rhythm, or VT).
PVCs that look the same in the same lead are called unifocal PVCs. These PVCs originate from a single ectopic focus in the ventricles. PVCs that appear different from one another in the same lead are called multifocal PVCs (Figure 9-12). These PVCs usually originate from different ectopic sites, but sometimes may fire from a single site and are conducted along different routes in the ventricles, resulting in a QRS that differs in morphology in the same lead.
Box 9-2. Premature ventricular contraction (PVC): Identifying ECG features
Rhythm: Underlying rhythm usually regular; irregular with PVC
Rate: That of underlying rhythm (usually sinus)
P waves: None associated with PVC; P waves associated with the underlying sinus rhythm can occasionally be seen just before the PVC or after the PVC in the ST segment or T wave; usually these P waves are hidden in the QRS complex
PR interval: Not measurable
QRS complex: Premature QRS complex; wide (0.12 second or greater)
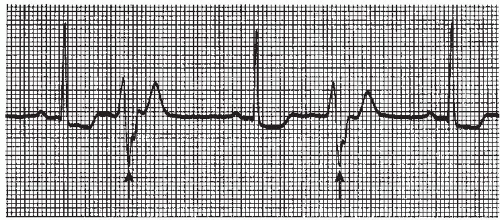
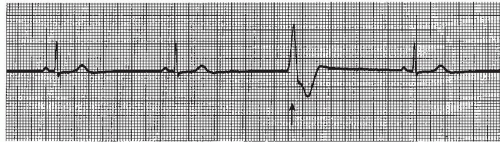
A PVC sandwiched between two normally conducted sinus beats, without greatly disturbing the regularity of the underlying rhythm, is called an interpolated PVC (Figure 9-13). The compensatory pause, usually associated with the PVC, is absent.
R-on-T PVC (Figure 9-14) is a term used to describe a PVC which falls on the down slope of the preceding T wave. This period corresponds to the relative refractory period of ventricular repolarization when the myocardium is in its most vulnerable state electrically. During this period, the myocardial cells have repolarized enough to respond to a strong stimulus. Stimulation of the ventricle at this time may precipitate repetitive ventricular contractions, resulting in VT or fibrillation.
PVCs are among the most commonly seen arrhythmias. PVCs may occur in individuals with a healthy heart, but are more common in people with coronary heart disease. PVCs are commonly caused by an increase in sympathetic tone from emotional stress; ingestion of substances such as alcohol, caffeine, or tobacco; mitral valve prolapse, myocardial ischemia or infarction; cardiomyopathy; congestive heart failure; hypoxia; electrolyte imbalances (especially hypokalemia); drug effects (digitalis, epinephrine, norepinephrine); as a reperfusion arrhythmia after thrombolytic therapy or angioplasty; or following insertion of invasive catheters into the heart, such as pacing leads or a pulmonary artery catheter.
Treatment of PVCs depends on the cause, the patient’s symptoms, and the clinical setting. Because occasional PVCs are a normal finding in healthy individuals, no treatment may be indicated, especially if the person is asymptomatic. Initially, a search should be made for possible reversible causes (such as oxygen for hypoxia; replacement of electrolytes; diuretics for heart failure; elimination of certain drugs; avoidance of alcohol, caffeine, or tobacco; and administration of antianxiety if indicated). Significant PVCs (more than 6 per minute, multifocal PVCs, paired PVCs, R-on-T PVCs, or PVCs in runs of 3 or more) should be treated with an antiarrhythmic medication, especially in the setting of acute MI or following cardiac surgery because of the increased risk of VT and VF in this setting.
On some occasions a ventricular beat may occur late instead of early. A late ectopic ventricular beat usually occurs after a pause in the underlying rhythm in which the dominant pacemaker (usually the sinus node) fails to initiate an impulse. If the ventricles are not activated by the sinus node, atria, or AV junction within a certain period of time, a focus in the ventricles may “escape” and pace the heart. These are called ventricular escape beats (Figure 9-15). The ventricular escape beat is a protective mechanism, protecting the heart from slow rates, and no treatment is required.
Ventricular tachycardia
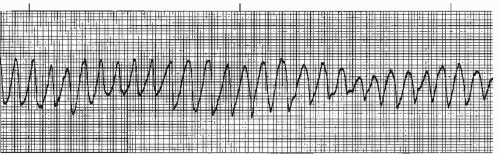
Ventricular tachycardia (VT) (Figures 9-16, 9-17, 9-18, 9-19 and 9-20 and Box 9-3) is an arrhythmia originating in an ectopic focus in the ventricles discharging impulses at a rate of 140 to 250 beats per minute. VT is most likely due to reentry in the ventricles, but can also be caused by enhanced automaticity of a focus in the ventricles or to triggered activity occurring during ventricular repolarization. VT occurs as a series of wide QRS complexes seen in short runs or as a continuous rhythm. Because of the ventricular origin of the impulse, no P waves are produced. The rhythm is usually regular, but may be slightly irregular. The ST segment and T wave slope in the opposite direction from the main QRS deflection. When the QRS complexes are of the same morphology in the same lead, the rhythm is termed monomorphic VT. When the QRS complexes differ in morphology in the same lead, the VT is called polymorphic VT.
Box 9-3. Ventricular tachycardia (VT): Identifying ECG features
Rhythm: Regular; can be slightly irregular
Rate: 140 to 250 beats/minute
P waves: No P waves are associated with VT.
PR interval: Not measurable
QRS complex: Wide (0.12 second or greater)
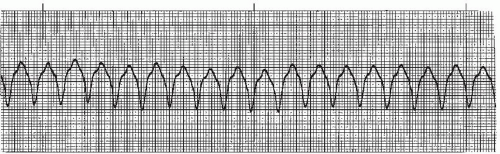 Figure 9-16. Ventricular tachycardia. Rhythm: Regular Rate: 150 beats/minute P waves: None identified PR interval: Not measurable QRS complex: 0.14 to 0.16 second. |
VT may occasionally occur at rates greater than 250 beats/minute. At such extreme rates the QRS complexes appear sawtooth in appearance and the rhythm is commonly referred to as ventricular flutter (Figure 9-17). Ventricular flutter is so rapid that there is virtually no cardiac output. Ventricular flutter is often a precursor to ventricular fibrillation.
VT usually occurs in patients with underlying heart disease. It may be preceded by significant PVCs (more than 6 per minute, paired PVCs, multifocal PVCs), but often occurs without preexisting or precipitating PVCs. The most common cause of sustained VT is coronary artery disease with prior MI. Other causes include myocardial ischemia, acute MI, cardiomyopathy, congestive heart failure, mitral valve prolapse, valvular heart disease, digitalis toxicity, electrolyte imbalances (especially hypokalemia and hypomagnesemia), myocardial contusion, mechanical stimulation of the endocardium by a pacing catheter or pulmonary artery catheter, as an effect of reperfusion following thrombolytic therapy or angioplasty, and drugs that increase sympathetic tone (epinephrine, norepinephrine, dopamine). Certain medications or conditions may prolong the QT interval, causing the ventricles to be particularly vulnerable to a type of polymorphic VT called torsade de pointes (Figure 9-19).
When VT lasts for less than 30 seconds it is called nonsustained VT. VT occurring in short runs of three or more consecutive PVCs at a rate of 140 to 250 beats per minute is considered a “run” or “burst” of nonsustained VT (Figures 9-11 and 9-18). Nonsustained VT, unless frequent, usually doesn’t cause symptoms, but it can progress into sustained VT. When VT lasts longer than 30 seconds, it is considered sustained VT. Sustained VT is a life threatening arrhythmia for two major reasons:
1. The rapid ventricular rate and loss of atrial kick reduce cardiac output. This reduction in cardiac output often compounds the already low cardiac output frequently seen in the diseased hearts in which VT tends to occur.
2. The rhythm may degenerate into VF or asystole.
Treatment is based on the patient’s presentation. An “unstable” patient refers to an individual who presents with symptoms such as hypotension, chest pain, shortness of breath, signs of decreased perfusion (cool, clammy skin; peripheral cyanosis; decreased level of consciousness; or a decrease in urine output). A “stable” patient refers to an individual with normal blood pressure, no chest pain, and no shortness of breath or signs of decreased perfusion. As part of the initial assessment you should check for a pulse. If there is not a pulse (pulseless VT), the rhythm must be treated as VF. If there is a pulse, protocols for stable VT and unstable VT are followed.
Treatment protocols: Stable monomorphic VT with pulse
▪ Amiodarone (150 mg in 100 mL D5W) is given as an intravenous piggy-back (IVPB) bolus over 10 minutes. An additional 150 mg IVPB bolus dose can be repeated in 10 minutes for resistant VT. Once the rhythm converts to a stable rhythm, an amiodarone maintenance infusion should be started to prevent reoccurrence of VT. The amiodarone maintenance infusion (900 mg in 500 mL D5W in a glass bottle) is started at 1 mg per minute for 6 hours, then decreased to 0.5 mg per minute for 18 hours. The total dose of amiodarone (IVPB bolus doses plus maintenance infusion) should not exceed 2.2 g in 24 hours. Oral amiodarone can be started once the maintenance infusion is completed. Elimination of the drug from the body is extremely long (half-life lasts up to 40 days).
▪ If the rhythm is unresponsive to amiodarone, sedate the patient and perform synchronized cardioversion beginning at 100 joules biphasic energy dose, increasing in a stepwise fashion with subsequent attempts.
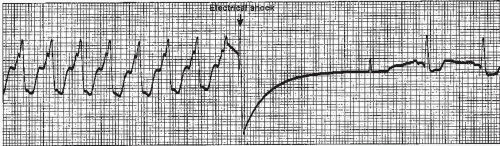
Some physicians prefer to skip drug therapy and go directly to synchronized cardioversion. Figure 9-20 shows cardioversion of VT to sinus rhythm.
Treatment protocols: Unstable monomorphic VT with pulse
▪ Sedate the patient (if conscious).
▪ Convert the rhythm using synchronized cardioversion beginning at 100 joules biphasic energy dose, increasing in stepwise fashion with subsequent attempts. Once cardioversion has converted the rhythm, a maintenance infusion of amiodarone is usually started at 1 mg per minute for 6 hours, then decreased to 0.5 mg per minute for 18 hours, followed by oral amiodarone once the maintenance infusion is completed.
Treatment of chronic, recurrent VT usually includes therapy with an oral antiarrhythmic. Patients who are refractory to a pharmacologic approach may require further evaluation, which could include specialized electrophysiologic testing and endocardial mapping with long-term options including the use of an implantable cardioverter defibrillator (ICD) or reentry circuit ablation. The ICD is a surgically implanted device developed to deliver an electric shock directly to the heart during a life-threatening tachycardia. Ablation (destruction) of the reentry circuit involves delivering short pulses of radiofrequency current through an intracardiac catheter. It produces a small burn that effectively blocks the part of the circuit supporting the reentrant-type wave.
Torsade de pointes ventricular tachycardia
Torsade de pointes (TdP) (Figure 9-19) is a form of polymorphic VT. This name is derived from a French term meaning “twisting of the points,” which describes a QRS complex that changes polarity (from negative to positive
and positive to negative) as it twists around the isoelectric line. TdP is an intermediary arrhythmia between VT and VF.
and positive to negative) as it twists around the isoelectric line. TdP is an intermediary arrhythmia between VT and VF.
TdP typically occurs when the QT interval of the underlying rhythm is abnormally prolonged, usually 0.5 second or greater. A prolonged QT interval or long QT syndrome (LQTS) is an abnormality of the heart’s electrical system. Although the mechanical function of the heart is entirely normal, the electrical problem is thought to be caused by changes in the cardiac ion channels that affect repolarization, causing a lengthened relative refractory period (vulnerable period) that puts the ventricles at risk for TdP and may result in sudden death.
Some causes of TdP VT include bradyarrhythmias (marked sinus bradycardia, third-degree AV block with a slow ventricular response); excessive administration of antiarrhythmics (quinidine, procainamide, disopyramide, amiodarone, sotalol); phenothiazines (prochlorperazine, chloropromazine, thioridazine); psychotropic medications (haloperidol, amitriptyline); electrolyte imbalances (especially hypokalemia, hypomagnesemia, hypocalcemia); liquid protein diets; central nervous system disorders (subarachnoid hemorrhage or intracranial trauma); and congenital LQTS.
The ventricular rate in TdP VT is extremely rapid and the patient usually becomes unstable very quickly. Recognition of TdP is critical not only because of the rapid deterioration of the patient but also because the treatment plan differs greatly from the treatment of monomorphic VT. Amiodarone, a drug used in treating monomorphic VT, can prolong the QT interval and make matters worse in this situation.
Treatment protocols: TdP VT
▪ The initial treatment should be immediate unsynchronized shock at 200 joules biphasic energy dose. Due to the variability in the QRS complexes in TdP, it might be difficult or impossible to reliably synchronize to a QRS complex. Although TdP is responsive to electrical therapy, the rhythm has a tendency to recur unless the precipitating factors are eliminated.
▪ Magnesium is the pharmacologic treatment of choice for TdP VT. Magnesium is usually very effective even in patients with normal magnesium levels. Magnesium acts as an antiarrhythmic and may terminate or prevent recurrent episodes of TdP. Give a loading dose of 1 to 2 g IV diluted in 10 mL D5W slowly over 5 minutes. This is followed by a 0.5 to 1 g/hour IV drip. A side effect of magnesium is hypotension, especially if administered rapidly. Magnesium also reduces neuromuscular tone and close monitoring of deep tendon reflexes is suggested.
▪ Potassium chloride (like magnesium) is a first-line therapy for TdP. Potassium is essential for maintenance of intracellular tonicity; transmission of nerve impulses; contraction of cardiac, skeletal, and smooth muscles; and maintenance of normal renal function. Depletion usually results from diuretic therapy, diabetic ketoacidosis, severe diarrhea, or inadequate replacement during prolonged parenteral nutrition therapy. Dosage of potassium depends on the serum potassium level, hospital protocols, and physician orders.
▪ Removing or correcting precipitating factors:
1. Bradycardia-induced — Discontinue drugs that decrease heart rate; overdrive pacing or isoproterenol infusion may be used to increase heart rate.
2. Drug-induced — Discontinue drugs that prolong QT interval.
3. Electrolyte-induced — Correct electrolyte abnormalities; magnesium and potassium are considered first-line therapy.
In treatment of congenital prolonged QT syndrome or recurrent TdP VT, an implantable defibrillator ICD can be used as prophylaxis.
Ventricular fibrillation
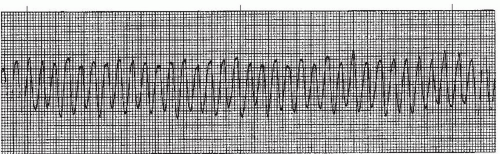
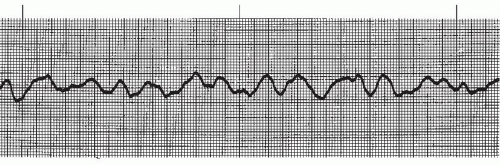
In ventricular fibrillation (VF) (Figures 9-21 and 9-22 and Box 9-4) a disorganized, chaotic, electrical focus in the ventricles takes over control of the heart. Organized ventricular depolarization and contraction do not occur (there is no QRS complex), but instead the ventricular muscle quivers and is often described as resembling a “bag of worms.” The ECG in VF shows characteristic fibrillatory waves that vary in shape and amplitude in an irregular and chaotic pattern.
VF with large amplitude waves is called coarse VF (Figure 9-21). If the VF waves are small, the rhythm is called fine VF (Figure 9-22). Coarse VF waves are generally more irregular than fine VF waves. Fine VF may resemble ventricular asystole and should be confirmed by examining the rhythm in different leads. The distinction between fine VF and coarse VF is significant because coarse VF usually indicates a more recent onset and is more likely to be reversed by early defibrillation. Fine VF usually indicates that the rhythm has been present longer and may require drug therapy and cardiopulmonary resuscitation (CPR) before defibrillation can be effective. Fine VF will progress to asystole unless the rhythm is treated.
Box 9-4. Ventricular fibrillation (VF): Identifying ECG features
Rhythm: None (P wave and QRS complex are absent)
Rate: None (P wave and QRS complex are absent)
P waves: Absent; wavy, irregular deflections seen, varying in size, shape, and height and representative of quivering of the ventricles instead of contraction; deflections may be small (described as fine VF) or large (described as coarse VF)
PR interval: Not measurable
QRS complex: Absent
VF is the most common cause of cardiac death in patients with acute MI. Other causes include myocardial ischemia, hypoxia, cardiomyopathy, electrolyte imbalances (especially hypokalemia and hypomagnesemia), digitalis toxicity, excessive doses of antiarrhythmics, cardiac trauma, and mitral valve prolapse. VF may be preceded by significant PVCs or VT, but it may also occur spontaneously without precipitating rhythms. VF may also occur during anesthesia, cardiac catheterization procedures, pacemaker implantation, placement of a pulmonary artery catheter, or after accidental electrocution.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree