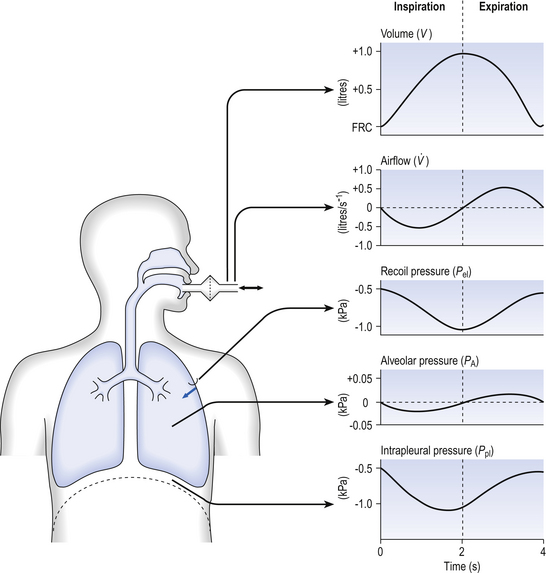
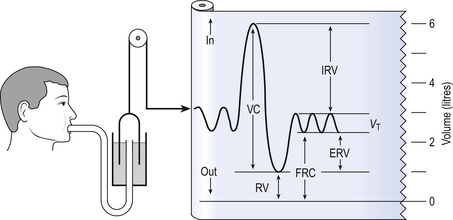
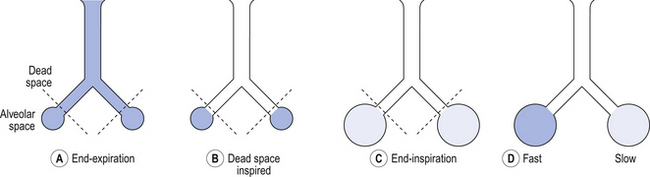
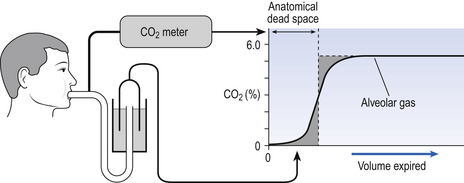
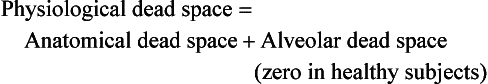5 In respiratory medicine ventilation is the rate of flow of air into or out of the lungs, and results from the expanding and contracting of the lungs by the changes in intrapleural pressure described in Chapter 4 (pp. 41, 42) and illustrated in Figure 5.1. We all know we can consciously alter the volume of our lungs, breathing in or breathing out more than normal: what is frequently not realized is that we cannot totally empty our lungs. The anatomy (size) of an individual’s chest, the elasticity of his lungs and chest wall and the strength of his respiratory muscles determine these volumes. Changes in lung volume can easily be measured using a spirometer, as illustrated in Figure 5.2. This instrument, which comes in many forms, consists of a closed space from which the subject breathes. In the type illustrated a hollow bell is supported in a trough of water; as the subject breathes in, air is drawn from the bell and it sinks slightly; when he breathes out the bell rises. Movements of the bell are recorded as changes in lung volume on a moving chart. Except for RV and FRC (which depends on RV), these volumes can be measured using a spirometer in the living subject. If the lungs are taken out of the body and allowed to collapse there will still be a little air left in them: the minimal air; these lungs will float (see ‘lights’ Chapter 2, p. 19). The lungs of a stillborn baby who has not taken a breath will not float because they contain no air; this test is important in forensic investigations. The names of these volumes and their abbreviations are intimidating to students, but reference to Figure 5.2 should make all clear. • body size – all are larger in large people. • age – all volumes are smaller in children, only partially due to their smaller body size. In old age VC is decreased and RV increased because of degenerative changes. • sex – all volumes are smaller in women than in men the same size. • muscle training – increases all the lung volumes and allows greater maximal ventilation during exercise. • disease – changes in these lung volumes from the normal values, which have been measured in numerous extensive surveys, are used in the diagnosis of many diseases of the lungs and respiratory system. Lung disease changes many of the lung volumes in Figure 5.2. It is usual, for diagnostic purposes, to exaggerate these changes by stressing the respiratory system by asking the patient to breathe in as deeply as he can and out as hard as he can for the single breath of a test. The forced expiratory volume in 1 second (FEV1) is frequently abbreviated to forced expired volume (FEV), but is still the same creature: the volume of air forced out in the first second of such a test. Similarly, forced vital capacity is the total volume of air a patient can breathe out after a maximal inspiration. It is usual to express FEV1 as a percentage of FVC: this takes into account the fact that larger people normally have larger lungs and therefore a larger FVC. Often VC is used in place of FVC in this ratio. Diseases of the thoracic cage, such as ankylosing spondylitis, diseases of the nerves and muscles of respiration, e.g. poliomyelitis, diseases that restrict expansion of the lungs, such as fibrosis, or diseases that cause airway collapse during expiration all limit these spirometric measurements. Examples of the modifications produced by diseases of the lungs on spirometric traces are shown in Chapter 11, and can be summarized in a very general way as follows: 0 = no change; + =increase; − =decrease. NB: some of these changes are not seen to any degree until the disease is very advanced. These considerations of the various volumes that make up breathing still give an impression of uniformity of distribution which is far from true. In Chapter 2 we described the anatomy of the bronchial tree as blind-ended sacks connected to the outside through a system of tubes. Some thought enables us to see that the composition of gas in this arrangement may be different at its entrance (the lips) from that at its ends (the alveoli) – differences in series with each other; also, the composition in different alveoli may be different – differences in parallel; or a combination of both. Differences in the composition of air in different parts of the lung depend largely on how well that part is ventilated and how much gas exchange between air and blood and blood and air takes place there. In the ideal situation just the right amount of both air and blood are supplied to a particular region, so there is no ‘waste’ of either. One particular kind of inequality between air and blood supply is known as dead space. Essentially all exchange of gas between air and blood only takes place at the alveolar surface. The system of tubes connecting this surface to the atmosphere takes little part in this exchange and can be considered anatomical dead space. These tubes are essential to bring air to the respiratory surface, but ventilating these connecting tubes is an inescapable waste of effort as far as gas exchange is concerned. To understand anatomical dead space you must understand that the lungs fill and empty in a sequential fashion (Fig. 5.3). At the end of inspiration the contents of the alveoli have been diluted by inspired room air, which now also fills the anatomical dead space (Fig. 5.3C). As the lungs then empty during expiration, the rule of ‘last in first out’ applies and the dead space containing unmodified room air is exhaled first. At the end of expiration the anatomical dead space is filled with alveolar air, and this partly used air is inhaled first in the next inspiration (Fig. 5.3A,B). If some regions of the lung expand before others in the process of inspiration they will receive an inappropriately large part of this dead space gas, and the regions receiving air later in inspiration will receive more fresh air (Fig. 5.3C,D). So the timing of inflation of a part of the lung during inspiration will affect the composition of the gas it receives. This type of dead space is called ‘anatomical’ because it measures the anatomical volume of the conducting airways. The strict definition of anatomical dead space is ‘the volume of an inspired breath which has not mixed with the gas in the alveoli’. Because gas exchange effectively only takes place in the alveoli there is no CO2 excreted into the dead space, and a scientist called Fowler pointed out that anatomical dead space can be measured as the volume of expired gas leaving the mouth and nose before CO2 appears at the lips (Fig. 5.4). We will see in a little while that this ‘cunning plan’ for measuring anatomical dead space is fraught with difficulty, mainly because the alveolar gas appearing at the lips does not have the constant composition shown in Figure 5.4. More often there is a considerable slope, particularly when the subject is breathing vigorously, or when alveoli empty at different rates. This makes deciding where to draw the vertical line difficult. It would be wrong to think of alveolar dead space as an absolute term, i.e. to imagine areas of the lung that are supplied with air by breathing but which have absolutely no blood supply to exchange O2 and CO2 with this air. We will see in Chapter 7 that most of the lung is ‘on target’, getting lots of blood to regions that are well ventilated and less blood to poorly ventilated regions. This ‘
VENTILATION OF THE RESPIRATORY SYSTEM
THE IMPORTANCE OF ITS LACK OF UNIFORMITY IN DISEASE
Introduction
Spirometric abnormalities in disease
Variable
Restrictive disease
Obstructive disease
FVC
− −
−
FEV1
− −
− −
FEV1/FVC
0
− −
FRC
−
+
RV
−
+
TLC
−
+
Uneven distribution
Dead space
Alveolar dead space in disease
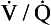 matching’ is of course very important, and it is the major defect in diseases as varied as emphysema and pulmonary fibrosis, in which areas of the lung may be expanded, but, because there is only a slow change of air within that space, are poorly ventilated.
matching’ is of course very important, and it is the major defect in diseases as varied as emphysema and pulmonary fibrosis, in which areas of the lung may be expanded, but, because there is only a slow change of air within that space, are poorly ventilated.
VENTILATION OF THE RESPIRATORY SYSTEM: THE IMPORTANCE OF ITS LACK OF UNIFORMITY IN DISEASE

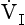 ) is:
) is: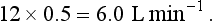
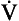 , has a dot over the V to show it is a rate. The volume breathed out is approximately equal to the volume breathed in (tidal volume, VT), therefore the net flow over a complete cycle is zero. This is not a very helpful way of expressing ventilation if we want to express changes in breathing, as the result of exercise or disease, for example. We therefore measure the flow in one direction only – conventionally the volume breathed out per minute (
, has a dot over the V to show it is a rate. The volume breathed out is approximately equal to the volume breathed in (tidal volume, VT), therefore the net flow over a complete cycle is zero. This is not a very helpful way of expressing ventilation if we want to express changes in breathing, as the result of exercise or disease, for example. We therefore measure the flow in one direction only – conventionally the volume breathed out per minute (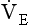 ) – to give us minute ventilation.
) – to give us minute ventilation.