Venous Thromboembolism
Samuel Z. Goldhaber
Venous thromboembolism (VTE) encompasses both deep vein thrombosis (DVT) and pulmonary embolism (PE). Diagnosis, management, and prevention of VTE can be standardized and adapted to critical pathways.
Cardiologists need to be adept in detecting DVT and in managing complicated cases that require catheter-directed thrombolysis, mechanical thrombectomy, or placement of an inferior vena caval filter. Furthermore, cardiologists should set the standard for recommending and implementing prophylaxis among their hospitalized patients and among patients on whom they consult preoperatively.
Cardiologists are often summoned to help diagnose suspected PE because of their familiarity with the differential diagnosis of chest pain and dyspnea, their ability to recognize clinical manifestations of acute pulmonary hypertension, and their facility with echocardiography for risk stratification. The cardiologist is often the specialist asked to manage high-risk patients with thrombolysis and, if necessary, suction catheter embolectomy. The cardiologist may also serve as the liaison with the cardiac surgeon who is summoned to perform urgent open surgical embolectomy.
Epidemiology
VTE is the third most common cardiovascular disease, after acute coronary syndrome and stroke (1). VTE spans a wide age range, from teenagers to the elderly. It strikes all socioeconomic groups in developed Western countries. However, rates of VTE in Japan are quickly catching up to those in North America and Europe (2).
Factor V Leiden is an autosomal dominant single-point genetic mutation that increases the likelihood of developing DVT or PE (3,4). This mutation also contributes to placental vein thrombosis and is associated with otherwise unexplained first trimester pregnancy losses (5). Racially, factor V Leiden is most commonly found in Caucasians, especially Northern Europeans (6). The mutation expresses itself with clinical venous thrombosis more frequently as patients age (7). Although factor V Leiden is the most thoroughly studied hereditary thrombophilia, other mutations with similar effects have been described, such as the prothrombin gene mutation (8,9,10).
Acquired risk factors warrant attention (11). These include long-haul air travel, women’s health issues (oral contraceptives, pregnancy, and hormone replacement therapy), obesity, cigarette smoking, hypertension, increasing age, and cancer. Certain combinations of genetic and acquired risk factors lead to a markedly increased likelihood of thrombosis, such as oral contraceptives in the setting of factor V Leiden.
Sorting out the contributions of heredity versus environment remains challenging. Widespread genetic testing will rarely change the management of patients with thromboembolic disease. Future studies will define more precisely the interaction between genetic thrombophilia and acquired risk factors for thrombosis.
For those who survive, DVT may result in lifelong disability with painful and disfiguring venous insufficiency of the legs. Chronic leg swelling, bulging varicose veins, and occasional ulceration may ensue, along with brownish skin discoloration at the medial malleolus. Venous insufficiency is surprisingly common. It can develop in as many as one-third of patients who have DVT. Often, it becomes apparent as a late complication, years after the initial thrombotic event.
Acute PE patients may develop chronic thromboembolic pulmonary hypertension within 6 weeks to several years. Chronic thromboembolic disease develops about 4% of the time (12). This is an upward revision from previous estimates of 1 in 500 cases. This devastating complication can cause profound dyspnea and lifestyle limitation that precludes ordinary walking and working outside the home. Patients with pulmonary hypertension are susceptible to sudden cardiac death and must cope with dyspnea at rest as well as with exertion.
There is also a huge psychological burden for many patients with VTE. They often are young and appear otherwise healthy. Yet their lifestyle is impaired by the constraints of long-term anticoagulation. They wonder whether their children or siblings will suddenly develop DVT or PE. Also, for those who discontinue anticoagulation, they can never be certain about whether VTE will recur.
VTE is underdiagnosed because it is often asymptomatic. The best clue to detection may be the clinical setting and assessment of predisposing risk factors (13). These include: “medical risk factors” such as prior VTE, cancer, surgery, trauma, bedrest, or immobilization, which are beyond control of the patient (Table 22-1), as well as “environmental factors,” such as obesity, cigarette smoking, hypertension, oral contraceptives, pregnancy, hormone replacement therapy, and long-haul air travel (Table 22-2).
VTE is often a chronic illness. Overall, about 30% will suffer
recurrence over the ensuing 10 years unless anticoagulation is continued.
recurrence over the ensuing 10 years unless anticoagulation is continued.
Table 22-1. Medical Risk Factors | ||||
|---|---|---|---|---|
|
Diagnosis
Deep Vein Thrombosis
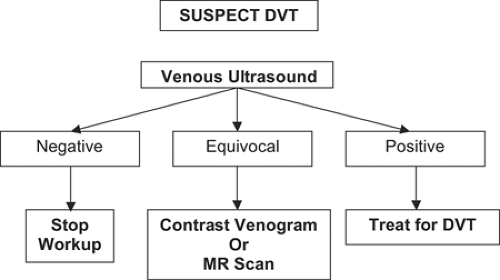
Figure 22-1 summarizes a critical pathway for DVT diagnosis. For DVT patients, the most common chief complaint is a cramp or “charley horse” in the lower calf that does not abate and gradually worsens after several days. Discomfort, at first intermittent, becomes persistent. Swelling may then ensue. Occasionally, erythema accompanies the leg edema. Erythema often suggests concomitant superficial venous phlebitis with saphenous vein involvement or coexisting cellulitis.
Unexplained arm edema may herald the presence of upper extremity DVT (14,15). This condition occurs most commonly in two divergent and contrasting populations: (a) as a complication of a chronic indwelling central venous catheter, or (b) in otherwise healthy individuals who have been exerting themselves with activities such as weight lifting.
Do not immediately jump to the diagnosis of DVT. Keep in mind the need to maintain a differential diagnosis (Table 22-3). Sudden, excruciating calf discomfort is most likely due to a ruptured Baker’s cyst. Fever and chills usually suggest cellulitis rather than DVT, though DVT may be present concomitantly.
When detected early after onset, the physical findings of DVT may be minimal, consisting of mild palpation discomfort in the lower calf. If the DVT propagates proximally because it is not recognized at an early stage, one might find massive thigh swelling and marked tenderness when palpating the inguinal area over the course of the femoral vein. Such patients often have difficulty walking and may require a cane, crutches, or a walker. If a patient has upper-extremity venous thrombosis, there may be asymmetry in the supraclavicular fossae or in the girth of the upper arms. There may also be a prominent superficial venous pattern over the anterior chest wall.
Table 22-2. Environmental Risk Factors | |||||
|---|---|---|---|---|---|
|
If the leg is diffusely edematous, DVT is unlikely. Much more common is an acute exacerbation of venous insufficiency due to postphlebitic syndrome.
When DVT is suspected, it is useful to estimate the clinical likelihood that DVT will be the final diagnosis. Clinical probability can be estimated using the formal Wells DVT Scoring System (16), but this approach is rarely used. Much more common is to estimate the likelihood by gestalt.
A few institutions, especially in Europe, favor stopping the DVT workup if the clinical probability is low and a D-dimer enzyme-linked immunosorbent assay (ELISA) is normal and not elevated. However, at most institutions in the United States, including Brigham and Women’s Hospital (BWH), we image with venous ultrasonography virtually all patients suspected of DVT. Of particular importance is proper interpretation of the ultrasound report. Unfortunately, the distal portion of the deep femoral vein is called the “superficial femoral vein.” Even though superficial is part of the name of this vein, it is a deep vein, and patients with superficial femoral vein thrombosis should be treated for DVT. They should not be discharged home with the misdiagnosis of superficial thrombophlebitis. “Superficial femoral vein thrombosis” can be a lethal misnomer (17).
Usually, the venous ultrasound examination is definitive in detecting or excluding DVT in the large upper extremity veins as well as the deep veins from the common femoral proximally to the calf veins distally (18). Occasionally, the imaging test is equivocal because of the patient’s body habitus, recent leg trauma or surgery, or profound edema that limits compression of vascular structures. Under these circumstances, consider pursuing other imaging (19).
Magnetic resonance imaging (MRI) can provide surprisingly precise, detailed information about the venous system. MRI is especially useful in assessing suspected pelvic vein thrombosis and in defining the extent of upper extremity vein
thrombosis (20). MRI can also help estimate the age of thrombus based on various “spin” characteristics of the image (21).
thrombosis (20). MRI can also help estimate the age of thrombus based on various “spin” characteristics of the image (21).
Table 22-3. Differential Diagnosis of DVT | |||||
|---|---|---|---|---|---|
|
Invasive contrast venography (22) is rarely performed nowadays as a diagnostic test. Of necessity, invasive contrast venography is the first step when catheter intervention is planned with catheter-directed thrombolysis, suction embolectomy, angioplasty, or stenting.
Pulmonary Embolism
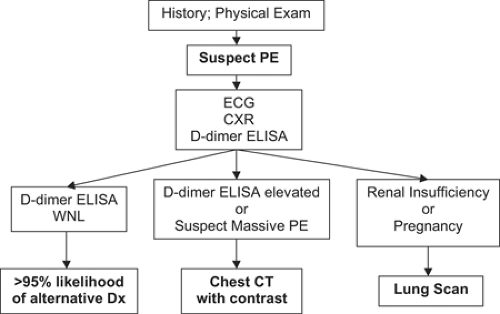
Figure 22-2 summarizes a critical pathway for PE diagnosis. Unexplained dyspnea and chest pain, often pleuritic, are the most common symptoms of PE. The Wells Scoring System for PE enjoys more popularity than the DVT Scoring System. However, I have never had a patient presented to me with incorporation of the Wells Scoring System unless I asked for it to be included. This Canadian system is used for clinical research purposes, so it is worth knowing. Nevertheless, gestalt is by far the most common way that clinical probability of PE is estimated.
The Wells Scoring System (23) is a point system where low probability for PE is less than 2 points, moderate probability is 2 to 6 points, and high probability is greater than 6 points. Seven variables comprised the point score: (a) clinical symptoms of DVT (3 points), (b) no alternative diagnosis (3 points), (c) heart rate greater than 100 beats per minute (1.5 points), (d) immobilization or surgery within the prior 4 weeks (1.5 points), (e) previous DVT or PE (1.5 points), (f) hemoptysis (1 point), and (g) cancer (1 point). The variable “no alternative diagnosis” is controversial because it is subjective, not objective, and is the “driving force” that makes the Wells Score an accurate predictor of the presence of absence of PE (24).
In a prospective observational study at BWH’s Emergency Department, there was a trend toward increasing accuracy of PE diagnosis with increasing clinical experience (25). However, the difference was not as great between an intern and an attending physician as one might have expected. Physicians were asked whether they thought PE was the most likely diagnosis. The frequency of true-positive assessments was 17% for interns and 25% for attending physicians.
Keep in mind that only about 10% of patients who undergo emergency imaging for PE actually have PE. Therefore, the astute clinician will formulate a differential diagnosis (Table 22-4), even when the manifestations of PE seem obvious.
The “classic findings” such as tachycardia, tachypnea, or hypotension actually represent unusual patients with extensive PE and impaired compensatory mechanisms. With improved imaging modalities, PE is with increasing frequency identified in normotensive patients who may appear anxious, but whose heart rate is less than 100 beats per minute and whose respiratory rate, if actually counted inconspicuously, is less than 16 breaths per minute.
Table 22-4. Adjunctive Measures for DVT Management | ||||||
|---|---|---|---|---|---|---|
|
Electrocardiography
Chest X-Ray
The chest x-ray (28) is useful for establishing alternative diagnoses such as pneumonia, congestive heart failure, or pneumothorax. PE patients may, however, present with an entirely normal chest x-ray.
Blood Tests
Arterial blood gases are unreliable and can be misleading (29,30). Specifically, patients who are otherwise healthy can present with large PE and yet maintain a high arterial PO2 and a normal arterial-alveolar oxygen gradient.
The only useful blood screening test is the plasma D-dimer ELISA (31). D-dimers are released in the presence of PE because
of endogenous fibrinolysis. Plasmin dissolves some of the fibrin clot from PE, and subsequently, D-dimers are released into the plasma. The D-dimers can be recognized by commercially available monoclonal antibodies. The D-dimer ELISA has a high negative predictive value for PE. This means that if the D-dimer ELISA is normal, it is extremely unlikely that PE is present (32).
of endogenous fibrinolysis. Plasmin dissolves some of the fibrin clot from PE, and subsequently, D-dimers are released into the plasma. The D-dimers can be recognized by commercially available monoclonal antibodies. The D-dimer ELISA has a high negative predictive value for PE. This means that if the D-dimer ELISA is normal, it is extremely unlikely that PE is present (32).
D-dimers are highly sensitive for the diagnosis of PE. This high sensitivity is crucially important in a screening test. However, D-dimers are nonspecific and will often be elevated in conditions that mimic PE such as acute myocardial infarction or pneumonia. They are also elevated in patients with cancer, in second or third trimester pregnancy, and in the postoperative state. Plasma D-dimer levels are usually elevated in hospitalized patients. Therefore, their contribution to diagnosis is greatest in outpatients suspected of PE; their use among hospitalized patients is minimal because the test results are rarely normal.
Imaging Tests
If the D-dimer ELISA is elevated, the next step is to order computed tomographic (CT) scanning of the chest. Chest CT scanning has revolutionized our diagnostic approach to suspected PE. By 2001, CT scanning was being used more often than lung scanning to investigate suspected PE (33). The clinical validity of using a CT scan to rule out PE is similar to that reported for conventional pulmonary angiography (34). This technology has evolved rapidly. The latest generation of scanners can diagnose submillimeter PE in sixth-order vessels. These thrombi are so tiny that their clinical significance is uncertain (35).
Chest CT scanning provides a satisfying dichotomous “yes” or “no” answer to whether PE is present. The CT examination can help identify the source of the clots in the legs, pelvis, or upper extremity. When PE is not present, the CT scan can help diagnose alternative pulmonary illnesses such as pneumonia, cancer, and interstitial lung diseases not apparent on the chest x-ray. The major limitation of chest CT scanning is the need to administer intravenous contrast.
Lung scanning has been the standard noninvasive imaging test for patients suspected of PE. It is highly specific when there is high probability for PE but it is not sensitive. Based on the Prospective Investigation of Pulmonary Embolism Diagnosis (PIOPED), more than half of the patients with PE proven by angiography had non-high probability lung scans (36). The principal disadvantage of lung scanning is that the majority of the scans are of intermediate probability for PE and frustrate the clinician because they do not provide a clear-cut answer to the diagnostic dilemma of whether PE is present.
When the lung scan is equivocal, leg vein ultrasonography may be helpful. If the venous ultrasound demonstrates DVT, this usually suffices as a surrogate for PE and the diagnostic workup can stop at this point. However, it is crucial to understand that as many as half of patients with PE will have no evidence of DVT, probably because the clot has already embolized to the lungs (37).
With a multislice CT scanner and a technologically adequate examination, the diagnosis of PE should be considered “ruled out” if the chest CT is negative (38). If a single-slice CT scanner shows no evidence of PE, it is still possible that multiple subsegmental PEs are present. Therefore, if clinical suspicion remains high, and if there is no access to a multislice CT scanner, and if a lung scan is equivocal, then invasive contrast pulmonary angiography should be considered (39).
MRI (40) is promising because it can combine imaging of the pulmonary arteries with functional and structural assessment of the right ventricle. MRI is also an excellent alternative among patients who are poor candidates for receiving intravenous contrast agent because of renal insufficiency or contrast dye allergy. For now, though, this technology is not sufficiently “mature” to be placed on a diagnosis critical pathway.
Initial Therapy
Deep Vein Thrombosis
The treatment of DVT has changed dramatically. DVT management used to require a 5-day hospitalization with intravenous unfractionated heparin administered as a bridge to warfarin. The administration of intravenous heparin required an initial intravenous bolus followed by a continuous infusion. The dose was titrated to achieve an activated partial thromboplastin time (aPTT) two to three times control (41). Generally, this corresponded to an aPTT between 60 and 80 seconds.
This standard approach to therapy was problematic. First, most patients were underdosed with heparin so that it required 24 to 48 hours to achieve therapeutic levels. There would be multiple changes in the dose of the continuous intravenous heparin infusion, based on emergency (“STAT”) aPTT values. These changes in dosing often led to medication errors (42). They were also extremely inconvenient and required additional physician and nurse time, often in the middle of the night. Although a robotic dosing system has been successfully tested (43), the need for traditional heparin appears to be limited to patients who are massively obese or in renal failure or who have an abhorrence of injections. Exposure to unfractionated heparin also increased the possibility of developing heparin-induced thrombocytopenia (44).
Now, however, most patients receive acute DVT treatment as outpatients or with an overnight hospital stay. In the United States, immediate anticoagulation is usually achieved with the low-molecular-weight heparin enoxaparin. This strategy is safe (45) and cost effective (46). Other U.S. Food and Drug Administration (FDA)-approved regimens include the low-molecular-weight heparin tinzaparin and the anti-Xa pentasaccharide fondaparinux.
Enoxaparin has received FDA approval for DVT treatment, as a “bridge” to warfarin because of its superior efficacy and safety compared with unfractionated heparin. Low-molecular-weight heparin is administered as a fixed dose according to weight (1 mg/kg twice daily or, among hospitalized patients, 1.5 mg/kg once daily) and is cost effective because no routine laboratory testing is required. It has become the foundation of anticoagulation of DVT patients and can facilitate complete outpatient treatment of this disease among properly selected patients.
Like low-molecular-weight heparin, fondaparinux is administered by subcutaneous injection, and no laboratory monitoring is required (47). Fondaparinux requires a once daily subcutaneous injection, requires only one of three possible doses based on weight, and has never been reported to cause heparin-induced thrombocytopenia. For fondaparinux, patients weighing less than 50 kg receive 5 mg, 50-kg to 100-kg patients receive 7.5 mg, and patients weighing more than 100 kg receive 10 mg. The drug is available in prefilled syringes of 5 mg, 7.5 mg, or 10 mg.
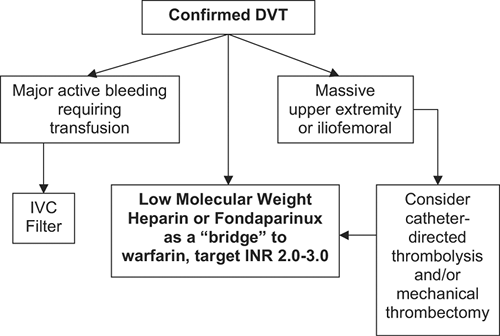
Even those patients who require prolonged hospitalization (Fig. 22-3) can usually be managed with low-molecular-weight heparin or fondaparinux until they achieve a stable and therapeutic level of warfarin, based upon a target International Normalized Ratio (INR) of 2.0 to 3.0. Overlap with the “bridging” anticoagulant should continue until two INRs are obtained in the therapeutic range. The success of an anticoagulation critical pathway depends primarily on reliable dosing and monitoring of warfarin. However, adjunctive measures for DVT management are also of paramount importance (Table 22-4).
Direct Thrombin Inhibitors
For patients with suspected or proven heparin-induced thrombocytopenia, neither unfractionated heparin nor low-molecular-weight heparin can be safely administered. The only FDA-approved alternative is the use of direct thrombin inhibitors, either argatroban or lepirudin (48). Argatroban, metabolized primarily by the liver, is particularly useful for patients with renal insufficiency. Lepirudin, metabolized primarily by the kidneys, is particularly useful for patients with hepatic dysfunction.
Catheter-Directed Interventions
The indications for DVT thrombolysis are uncertain and controversial (49). DVT thrombolysis should theoretically restore venous valve patency and function, thereby preventing the development of venous insufficiency and the postthrombotic syndrome. However, this hypothesis has not been proven. In addition, DVT thrombolysis may be especially useful in patients who have developed an upper-extremity thrombosis due to a long-term indwelling central venous catheter. For example, a patient may require completion of additional courses of chemotherapy or may need intravenous hyperalimentation.
At BWH, we advise DVT catheter-directed thrombolysis for young, otherwise healthy patients who have massive iliofemoral DVT with marked leg swelling, leg tenderness, and difficulty walking (Fig. 22-3). For patients with large DVT who require intervention in addition to anticoagulation, a combination of catheter-directed thrombolysis and catheter-assisted thrombectomy is usually utilized.
Chronic Venous Insufficiency
Venous insufficiency can first become clinically apparent several years after the initial DVT (50). The pathophysiological explanation is damage to the venous valves of the legs. Late onset of venous insufficiency is a separate problem from recurrent DVT. Wearing 30- to 40-mm Hg below-knee vascular compression stockings while ambulatory has proved effective in randomized clinical trials (51). Prescribing vascular compression stockings will halve the rate of subsequent venous insufficiency.
Emotional Support
Many patients with VTE will appear healthy and fit. They may be burdened with fear about the possible genetic implications of DVT or PE. They will often feel overwhelmed when advised to continue lifelong anticoagulation. Patients in whom anticoagulation is discontinued after 3 to 6 months of therapy may feel vulnerable to a future recurrent VTE. In addition, family, friends, and peers might not understand the implications of DVT or PE. My nurse and I help provide emotional support by running a support group one evening every third week. We and our patients have also written responses to Frequently Asked Questions, which we posted on the following Web site: http://www.web.mit.edu.easyaccess1.lib.cuhk.edu.hk/karen/www/faq.html.
Pulmonary Embolism
Risk Stratification
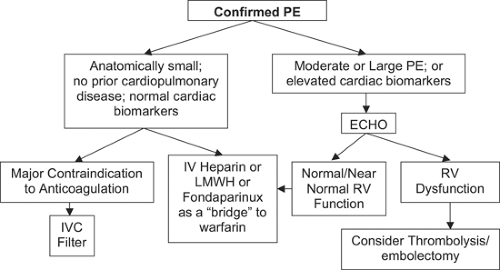
The clinical spectrum and severity of PE is wide. Prompt and accurate risk stratification are of paramount importance. After the diagnosis of PE is established, critical pathways are utilized to streamline, standardize, and optimize therapy (Fig. 22-4).
Most patients with PE will remain hemodynamically stable and will not suffer recurrent PE or develop chronic pulmonary hypertension as long as they receive adequate anticoagulation. If the PE is anatomically small, involving less than 30% of the lungs, then it is likely that right ventricular (RV) function will not be impaired (52), especially if there is no underlying cardiopulmonary disease.
Cardiac Biomarkers
Imaging the right ventricle is probably not necessary if the patient appears clinically stable and has normal levels of cardiac
biomarkers (53). Cardiac troponins are sensitive and specific biomarkers of myocardial cell damage. Elevations of troponin in PE patients are mild and of short duration compared with acute coronary syndromes. In acute PE, troponin levels correlate well with the extent of RV dysfunction (54).
biomarkers (53). Cardiac troponins are sensitive and specific biomarkers of myocardial cell damage. Elevations of troponin in PE patients are mild and of short duration compared with acute coronary syndromes. In acute PE, troponin levels correlate well with the extent of RV dysfunction (54).
Natriuretic peptides represent another class of cardiac biomarkers. The principal stimulus for synthesis and secretion of brain natriuretic peptide (BNP) is ventricular cardiomyocyte stretch (55). The prohormone, proBNP, has 108 amino acids. The biologically active BNP is a 32-amino acid peptide, with a plasma half life of 20 minutes. The remaining part of the prohormone, N-terminal (NT)-proBNP, has 76 amino acids and a half life of 60 to 120 minutes. The major role of cardiac biomarkers in risk stratification is to identify low-risk patients who do not require imaging of the right ventricle. Patients with normal levels of troponin and BNP are low risk. A simple risk stratification algorithm for PE patients is to employ either troponin or NT-proBNP testing as an initial step. Echocardiography should then be obtained if elevated biomarker levels are found (56). Echocardiography is not needed if both troponin and BNP (or pro-BNP) are normal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree