Venous Thromboembolism
Venous thromboembolism (VTE) is a common disease that includes both pulmonary embolism (PE) and deep vein thrombosis (DVT). It is the third most frequently occurring cardiovascular condition after ischemic heart disease and cerebrovascular accidents in the United States. Approximately 1 million people develop DVT and 600,000 develop PE each year, and death from VTE has been estimated to occur in as many as 60,000 to 200,000 Americans annually.1
ESSENTIAL FACTS ABOUT VTE
It is important to recognize the natural history of VTE to more fully appreciate its short-term mortality and long-term morbidity.
 VTE is a recurrent disease with a risk of recurrence up to 30% at 10 years for unprovoked events.2
VTE is a recurrent disease with a risk of recurrence up to 30% at 10 years for unprovoked events.2
 PE is the third most common cause of hospital-related death and is the most common preventable cause of hospital death.3,4
PE is the third most common cause of hospital-related death and is the most common preventable cause of hospital death.3,4
 The mortality rate for PE without treatment is approximately 30%.5
The mortality rate for PE without treatment is approximately 30%.5
 Patients with an acute PE who have right ventricular (RV) dysfunction documented by a transthoracic echocardiogram (TTE) have a higher in-hospital mortality (14%) and short-term mortality (20%) rate at 3 months.6
Patients with an acute PE who have right ventricular (RV) dysfunction documented by a transthoracic echocardiogram (TTE) have a higher in-hospital mortality (14%) and short-term mortality (20%) rate at 3 months.6
 Chronic thromboembolic pulmonary hypertension (CTPH) develops in as many as 3.8% of all PE patients by 2 years after their initial event.7
Chronic thromboembolic pulmonary hypertension (CTPH) develops in as many as 3.8% of all PE patients by 2 years after their initial event.7
 Approximately 40% to 50% of all patients with an acute symptomatic DVT (proximal to the popliteal vein), who are asymptomatic for PE, will have a high-probability ventilation/perfusion scan.
Approximately 40% to 50% of all patients with an acute symptomatic DVT (proximal to the popliteal vein), who are asymptomatic for PE, will have a high-probability ventilation/perfusion scan.
 The most common long-term complication of DVT is the postthrombotic syndrome (PTS), characterized by chronic leg swelling, pain, and nonhealing venous stasis ulcers, which occurs in as many as 30% of all patients within 10 years after a documented DVT.8,9
The most common long-term complication of DVT is the postthrombotic syndrome (PTS), characterized by chronic leg swelling, pain, and nonhealing venous stasis ulcers, which occurs in as many as 30% of all patients within 10 years after a documented DVT.8,9
RISK FACTORS FOR VENOUS THROMBOEMBOLISM
Venous thrombosis results from the combination of acquired and hereditary causes. The most common acquired and hereditary risk factors (referred to as hypercoagulable states or thrombophilia) are shown in Tables 49.1 and 49.2.
TABLE
49.1 Acquired Risk Factors For VTE

TABLE
49.2 Hereditary Risk Factors for VTE
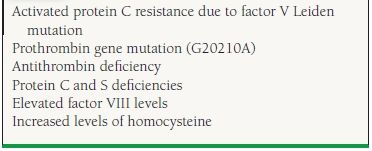
As many as 20% of white patients presenting with an idiopathic or unprovoked DVT are heterozygous for the factor V Leiden mutation, while 6% are heterozygous for the prothrombin G20210A mutation10 Both of these disorders are rare in the African and Asian populations.11,12 Other, less common hereditary hypercoagulable states include protein C and S and antithrombin deficiencies, hyperhomocystinemia, elevated levels of factor VIII, and dysfibrinogenemia.
DEEP VEIN THROMBOSIS: CLINICAL PRESENTATION AND DIAGNOSIS
Although VTE is considered to be one disease entity, the clinical presentation and diagnosis for DVT and PE are different. The characteristic symptoms for an acute DVT include leg or arm pain, swelling, increased skin temperature, and discoloration (erythrocyanosis), although these findings may be absent. Unfortunately, the clinical examination is often unreliable and the diagnosis is only confirmed in 20% to 40% of patients presenting with typical signs and symptoms.13 This is due in part to the varied differential diagnosis for DVT, which includes:
 Cellulitis
Cellulitis
 Arthritis, synovitis, myositis
Arthritis, synovitis, myositis
 Lymphedema
Lymphedema
 Arterial insufficiency
Arterial insufficiency
 Muscle ache or tear
Muscle ache or tear
 Baker cyst
Baker cyst
 Chronic venous insufficiency
Chronic venous insufficiency
 Systemic causes of edema (congestive heart failure [CHF], nephrotic syndrome, liver dysfunction, hypoalbuminemia)
Systemic causes of edema (congestive heart failure [CHF], nephrotic syndrome, liver dysfunction, hypoalbuminemia)
Clinical models have been developed to help diagnose an acute DVT. Wells et al. stratified outpatients presenting with a suspected DVT into low, intermediate, or high pretest probability categories based on a number of clinical “points.”14 According to their model, 3% of low, 17% of moderate, and 75% of high pretest probability patients were diagnosed with a DVT. Although it is not widely used, this model may be a helpful objective assessment tool for clinicians (Table 49.3).
TABLE
49.3 Clinical Feature Score According to Wells et al. Criteria
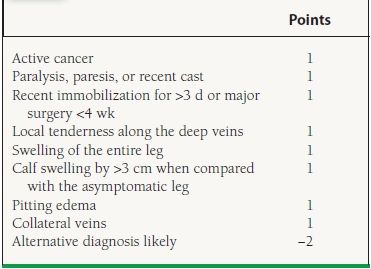
Risk score: low, <0 points; moderate, 1–2 points; high, >3 points. Adapted from Wells PS, Anderson DR, Bormanis J, et al. Value of assessment of pretest probability of deep-vein thrombosis in clinical management. Lancet. 1997; 350:1795–1878.
OBJECTIVE TESTING FOR DEEP VEIN THROMBOSIS
DVT can be confirmed using invasive and noninvasive studies as well as laboratory tests. These tests include:
 D-Dimer assay
D-Dimer assay
 Duplex ultrasonography
Duplex ultrasonography
 Venography
Venography
 Computed tomography venography (CTV)
Computed tomography venography (CTV)
 Magnetic resonance venography (MRV)
Magnetic resonance venography (MRV)
 Impedance plethysmography (IPG)
Impedance plethysmography (IPG)
The more commonly used diagnostic tests include a D-dimer assay and duplex ultrasonography.
D-Dimer
D-Dimer is a specific fragment of a fibrin clot whose presence indicates degradation of fibrin and serves as an indirect indicator for thrombotic activity. D-Dimer assay has been utilized to a great extent in the outpatient setting and emergency departments to rule out VTE, because of its high sensitivity and negative predictive value. An elevated D-dimer level, however, is not specific for VTE and can be seen in a variety of conditions, including pregnancy, infection, disseminated intravascular coagulation (DIC), hemorrhage, malignancy, liver disease, surgery, trauma, cardiac or renal failure, acute coronary syndrome (ACS), and acute nonlacunar stroke. It is important to remember that not all D-dimer assays are alike and that it can be measured using a number of different methods. A recent analysis found that the Enzyme-linked immunosorbent assay (ELISA) and quantitative rapid ELISA tests were superior to other methods.15
Most physicians feel that anticoagulation can be withheld from patients suspected of acute VTE in the outpatient setting if the D-dimer assay is negative. It is extremely important, however, for clinicians to know the sensitivity and specificity of their hospital’s D-dimer assay before making such a decision. If the clinician’s suspicion of VTE remains high despite a negative D-dimer assay, further imaging studies are recommended.
Duplex ultrasonography is a readily available, noninvasive modality that can be performed routinely in the hospital or outpatient setting or at bedside for a critically ill patient. It has replaced venography as the diagnostic method of choice for acute DVT and is the most accurate noninvasive test currently available. Duplex ultrasonography allows for direct visualization of the venous system. An inability to compress the vein with the ultrasound transducer is considered diagnostic for DVT. The other ultrasound findings that may help in the diagnosis of acute DVT are listed in Table 49.4.
TABLE
49.4 Ultrasound Characteristics of Acute DVT

Physicians must recognize that duplex ultrasonography is very operator dependent. Its sensitivity is approximately 95% and its specificity 96% in symptomatic patients with a proximal DVT, but it is less reliable in asymptomatic patients, those with thrombus above the inguinal ligament, or those with calf vein thrombosis. The sensitivity and specificity for isolated calf vein thrombosis approaches 60% to 70%.16
In a study involving 375 patients, the validity of withholding anticoagulation in patients with a negative ultrasound and a low clinical suspicion for DVT was examined.17 Only three patients who had anticoagulation withheld developed a new VTE event. Two patients developed an isolated calf vein DVT and one patient a proximal DVT (total of 0.8%). No patient developed a PE at the 3-month follow-up.
Duplex ultrasonography may also be useful in patients suspected of an acute PE. If the arms or legs are positive for an acute DVT, further confirmatory studies may be unnecessary in most patients, assuming that would not change the management of the patient.
Venography
The venogram is an invasive procedure now replaced for the diagnosis of DVT by duplex ultrasonography. An intraluminal-filling defect must be seen in at least two different projections for confirmation. A venogram should be considered in the appropriate clinical setting or when other tests are nondiagnostic. Despite its clinical utility, complications such as contrast allergy and postprocedural acute DVT should not be overlooked. The latter complication has been reported to occur in approximately 1% to 2% of all patients.
Other Diagnostic Testing Options
CTV of the legs can be performed in conjunction with a computed tomographic pulmonary angiogram (CTPA) of the chest used to rule out PE. Although more radiation is required, no additional contrast is needed and imaging of the more proximal leg veins (iliacs), pelvic veins, and the inferior vena cava (IVC) is possible. However, recent studies showed that routine CTV of the pelvis during CTPA does not significantly improve the detection of VTE and therefore should not be performed routinely in all patients being evaluated for PE.18,19
MRV imaging can also be utilized to diagnose DVT. Its sensitivity and specificity has been reported to be >95% when compared to standard venography for the diagnosis of a proximal DVT, although outcome data are lacking. It has several advantages, including (a) detecting pelvic, iliac, and IVC thrombosis and (b) no need for ionizing radiation. Potential drawbacks include lack of availability, high cost, reader expertise, difficulty with morbidly obese patients, and the presence of metallic objects (stents or other hardware) in the area of interest. The MRV modality may be beneficial in pregnancy when there is a high clinical suspicion for an IVC, pelvic, or iliac vein DVT that is not detectable with duplex ultrasonography, or for patients with an allergy to contrast dye. However, this imaging technique should not be used in patients with acute or chronic renal insufficiency to prevent the adverse effect of nephrogenic systemic fibrosis (NSF).20
IPG has largely been replaced by duplex ultrasonography at hospitals in the United States.
PULMONARY EMBOLISM: CLINICAL PRESENTATION AND DIAGNOSIS
Autopsy studies continue to demonstrate that most fatal cases of PE are unrecognized or not diagnosed.21 Patients presenting with PE often have nonspecific signs and symptoms, making the diagnosis more difficult and frequently overlooked. In a review of the most common signs and symptoms of patients presenting with an acute PE without underlying cardiopulmonary disease, dyspnea was most common, followed by pleuritic chest pain. These manifestations are valuable clues to the diagnosis in this patient population. However, in the individual with heart or lung disease, they may be mistaken for symptoms of the underlying disease process. Other signs and symptoms of an acute PE include cough, leg swelling, thrombophlebitis, hemoptysis, palpitations, wheezing, angina-like pain, apprehension, and fever.22.
Patients may present with a massive or submassive PE, or they may be entirely asymptomatic. Patients who have a massive PE (systolic arterial pressure <90 mm Hg) present with circulatory collapse and shock or syncope. Fortunately, this is not a common manifestation, representing only in about 8% of all patients.23 Acute shortness of breath, with tachycardia, chest pain, tachypnea, and cyanosis, may be the result of a submassive PE (pulmonary hypertension or RV dysfunction without arterial hypotension or shock). Patients with acute PE may also be entirely asymptomatic, especially in the postoperative period. Because of the wide variety of clinical presentations, both noninvasive and invasive diagnostic methods may be necessary to confirm the diagnosis. The differential diagnosis of PE includes:
 Unstable angina, myocardial infarction (MI)
Unstable angina, myocardial infarction (MI)
 Pneumonia
Pneumonia
 Chronic obstructive pulmonary disease (COPD), bronchitis
Chronic obstructive pulmonary disease (COPD), bronchitis
 CHF
CHF
 Pericarditis
Pericarditis
 Pneumothorax
Pneumothorax
 Costochondritis
Costochondritis
The diagnosis of PE should start with establishing the clinical probability. Wells et al. stratified the clinical features of PE into “points” (similar to their DVT criteria), and their model is useful as an objective tool to assess the pretest probability of an acute PE (Table 49.5).24 In their model, the probability of an acute PE with >6 points (high risk) was 78.4%, while that for 2 to 6 points (moderate risk) was 27.8% and for <2 points (low risk), it was 3.4%. The combination of a negative D-dimer and low to moderate pretest probability has a very high negative predictive value (~99%). In validation study using Wells criteria in combination with negative D-dimer, only 0.5% of patients with low to moderate pretest probability for PE later developed nonfatal VTE.25 D-Dimer has limited value in patients with a high clinical suspicion for PE,26 and the clinician should proceed directly with multidetector CTPA scan.
TABLE
49.5 Wells et al. Clinical Prediction for PE
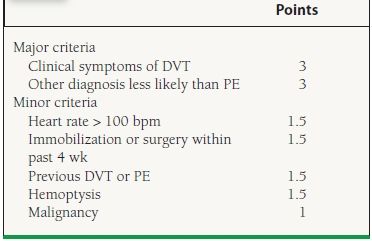
Risk score: low probability < 2, moderate probability 2–6, high probability > 6. Adapted from Wells PS, Anderson DR, Rodger M, et al. Derivation of a simple clinical model to categorize patients probability of pulmonary embolism: increasing the models utility with the SimpliRED D-dimer. Thromb Haemost. 2000;83:416–420
OBJECTIVE TESTING FOR PULMONARY EMBOLISM
Traditional tests used to assist a physician faced with the presumptive diagnosis of an acute PE include a chest x-ray, electrocardiogram (ECG), and an arterial blood gas.
The chest x-ray may be more helpful to rule out pneumonia, a pneumothorax, or a malignancy. The most common x-ray features of acute PE are consolidation, a pleural effusion, atelectasis, Hampton hump (wedge-shaped opacity along the pleural surface), Westermark sign (oligemia), and Palla sign (an enlarged right descending pulmonary artery).27 These latter three classic radiographic findings are rarely seen, however.
An ECG may exclude cardiac causes that mimic PE, such as an MI or a pericarditis. ECG findings suggestive of a PE include sinus tachycardia, new-onset atrial fibrillation or flutter, right bundle branch block, right-axis deviation, and nonspecific ST–T-wave changes. The classic finding of S1Q3T3 indicates acute cor pulmonale but is seen in <10% of all patients.27 Evidence of acute RV myocardial injury may also be seen, manifest as ST-segment elevation isolated to lead V1 and, at times, extending to lead V2.
PE must not be excluded based on either a normal arterial blood gas or a normal alveolar–arterial gradient (A-a gradient). In several studies, up to 20% of patients had normal oxygen levels and A-a gradients despite angiographically proven PE.28
Computed Tomography Pulmonary Angiography
CTPA not only allows direct visualization of the thrombus but also has great value in excluding other diseases, including an aortic dissection, pneumonia, or malignancy.
Over the last decade, multidetector spiral computed tomography (CT) has become the standard imaging technique for diagnosis of PE. Earlier reports suggested that a single row detector CTPA is both highly sensitive and specific for the diagnosis of a central PE (main, lobar, or segmental pulmonary arteries) but insensitive to the diagnosis of a subsegmental event, with a potential to miss smaller emboli. However, the use of multidetector CTPA has greatly increased its sensitivity and specificity for diagnosis of small peripheral or subsegmental PEs. A recent study suggested that CTPA is more sensitive than a ventilation/perfusion (V/Q) scan for the diagnosis of PE.29 In a meta-analysis of 23 studies involving 4,657 patients with a negative multidetector CT who did not receive anticoagulation therapy, the incidence of VTE was only 1.4%.30
CTPA can also assist in risk-stratifying patients with acute PE by identifying patients with an enlarged RV. RV enlargement on multidetector CT (defined by RV/left ventricular [LV] diameter ratio > 0.9) has been shown to predict doubling of mortality in the 30 days following diagnosis.31The major disadvantage of CTPA is the risk of contrast-induced nephropathy and radiation exposure.
The V/Q scan was long considered one of the most useful aids to diagnose acute PE. The PIOPED trial (prospective investigation of pulmonary embolism diagnosis) combined low, intermediate, or high preclinical suspicion with a normal-, low-, intermediate-, or high-probability V/Q scan.32A normal V/Q scan effectively excluded the diagnosis of an acute PE, whereas if the clinical suspicion and the perfusion scan showed high probabilities, the diagnosis was very likely. Ventilation/perfusion scans interpreted as low or intermediate probability were considered nondiagnostic and required further testing to confirm or exclude an acute PE.
In the PIOPED trial, 88% of patients with a high clinical suspicion and high-probability V/Q scan had acute PE confirmed by pulmonary angiography. Among patients with a low-probability V/Q scan, angiographically proven PE was identified in 40% and 4% of patients with a high and low preclinical suspicion, respectively.
Unfortunately, in as many as 75% to 80% of all PIOPED patients, no definitive diagnosis could be made because studies were interpreted either as low or intermediate probability.
Ventilation/perfusion scanning has been replaced by multidetector CTPA and currently is considered a second-line modality in the diagnosis of PE. However, V/Q scan remains a valuable tool in the diagnosis of acute PE in patients with a normal chest x-ray or patients who cannot undergo CTPA (contrast allergy, renal insufficiency, or pregnancy)
Pulmonary Angiography
Pulmonary angiography remains the reference standard for which most studies are compared in the diagnosis of PE, despite the fact that it is not universally available, is invasive, and is costly. The definitive diagnosis of acute PE requires the presence of an intraluminal-filling defect in at least two views or demonstration of an occluded pulmonary artery. It is not without complications, and morbidity of 5% and mortality of 0.5% were reported in the PIOPED trial.32
Echocardiography (Transthoracic and Transesophageal)
Abnormal TTE findings in acute PE include RV dilatation, RV hypokinesis, interventricular septal flattening or paradoxical motion, decreased inspiratory collapse of IVC, pulmonary artery hypertension and pulmonary artery dilatation, tricuspid regurgitation, patent foramen ovale (PFO) and rarely direct visualization of thrombus. The finding of akinesia of the mid-free RV wall with relative sparing of apex is referred to as McConnell sign. This sign was found in one study to have 94% specificity and 71% positive predictive value for the diagnosis of acute PE.33
Hemodynamically unstable patients generally suffer severe RV dysfunction; however, RV hypokinesis presents in only 40% of patients with normal systemic pressure. A TTE alone cannot be used to diagnose PE but is a useful tool for risk stratification and identifying patients with acute PE who may have a poor prognosis. RV dysfunction on TTE has been associated with increased mortality among patients with acute PE. In a retrospective study, patients with an RV/LV ratio ≥0.9 were 2.6 times more likely to die during hospitalization independently of other risk factors.34
Transesophageal echocardiogram (TEE) can be used as a rapid bedside test to allow direct visualization of the main pulmonary arteries and should be considered in the hemodynamically unstable patients who cannot be moved to undergo a CTPA scan or pulmonary angiogram.35
In PIOPED III, magnetic resonance angiography (MRA) had insufficient sensitivity and a high rate of technically inadequate images. Addition of MRV to MRA improves sensitivity; however, 52% of patients in the study had a technically inadequate study. At the current time, an MRA/MRV should be considered only at those centers with experience with this modality and only for patients for whom standard tests are contraindicated.36
Cardiospecific Biomarkers in Pulmonary Embolism
Cardiospecific biomarkers, including cardiac troponin and brain natriuretic peptide (BNP), have become useful in the risk stratification strategy of patients with an acute PE. Elevation in troponin levels and BNP correlate with the presence of RV dysfunction and appear to be independent risk factors for poor or fatal outcome. In a meta-analysis of 1985 acute PE patients, any elevation in troponin was found to confer a fivefold increase in short-term mortality.37 A troponin level is considered the single most useful blood test for the risk stratification of patients with acute PE, while a normal BNP has a negative predictive value of 97% to 100%.38,39
KEY POINTS IN DIAGNOSIS OF VTE
 Clinical prediction rules should be used to estimate the pretest probability for DVT and PE.
Clinical prediction rules should be used to estimate the pretest probability for DVT and PE.
 In patients with a low to moderate pretest probability for DVT or PE, a negative high sensitivity D-dimer has a very high negative predictive value (indicating a very low likelihood of VTE).
In patients with a low to moderate pretest probability for DVT or PE, a negative high sensitivity D-dimer has a very high negative predictive value (indicating a very low likelihood of VTE).
 Patients with a high pretest probability of VTE require additional diagnostic imaging studies.
Patients with a high pretest probability of VTE require additional diagnostic imaging studies.
 Duplex ultrasonography is accepted as the first-line diagnostic imaging study for DVT.
Duplex ultrasonography is accepted as the first-line diagnostic imaging study for DVT.
 Begin anticoagulation once suspect DVT (unless there is a contraindication for its use).
Begin anticoagulation once suspect DVT (unless there is a contraindication for its use).
 Patients with a high pretest probability of PE require additional diagnostic imaging studies such CTPA.
Patients with a high pretest probability of PE require additional diagnostic imaging studies such CTPA.
 Multidetector CTPA is accepted as the first-line diagnostic imaging study for acute PE.
Multidetector CTPA is accepted as the first-line diagnostic imaging study for acute PE.
 A V/Q scan is helpful if the chest x-ray is normal and a CTPA scan not possible or if the patient has renal insufficiency.
A V/Q scan is helpful if the chest x-ray is normal and a CTPA scan not possible or if the patient has renal insufficiency.
 Two D-echo or TEE is useful for the critically ill patient as a bedside test to help diagnose PE.
Two D-echo or TEE is useful for the critically ill patient as a bedside test to help diagnose PE.
 The presence of DVT on duplex ultrasonography is generally adequate for treatment of a PE.
The presence of DVT on duplex ultrasonography is generally adequate for treatment of a PE.
 Pulmonary angiogram remains the gold standard diagnostic imaging for PE if diagnosis uncertain.
Pulmonary angiogram remains the gold standard diagnostic imaging for PE if diagnosis uncertain.
 Troponins, BNP, and RV enlargement on echo or CT help predict outcome and are useful for risk stratification.
Troponins, BNP, and RV enlargement on echo or CT help predict outcome and are useful for risk stratification.
 Begin anticoagulation once suspect PE (unless there is a contraindication for its use) using risk stratification tools including echocardiography and troponins or BNP.
Begin anticoagulation once suspect PE (unless there is a contraindication for its use) using risk stratification tools including echocardiography and troponins or BNP.



