leg swelling +/- pitting edema
particularly if unilateral or involving the whole leg
objective measurement of circumference best taken 10 cm below the tibial tuberosity and compared to the unaffected leg
leg pain
distribution of the deep veins—within the calf and medial thigh
localized tenderness, particularly over a palpable cord or visible group of vessels, may be more in keeping with superficial phlebitis
redness and warmth
pallor or cyanosis (rarely)
may be associated with massive occlusive iliofemoral DVT
Homan’s sign (calf pain with forced dorsiflexion of the foot) is neither sensitive nor specific for DVT
chest pain, particularly pleuritic, sudden in onset and persistent
sudden onset of shortness of breath
preceding symptoms of DVT
tachycardia, cyanosis, signs of right-sided heart failure
hemoptysis (rare)
secondary to pulmonary infarction
syncope/shock (rare)
secondary to obstruction of right-sided cardiac output
VTE-unrelated interventions. Controlling or eradicating underlying risk factors can decrease the chances of treatment failure and thrombotic recurrence and can possibly shorten the duration of anticoagulation.
TABLE 21.1 RISK FACTORS FOR VTE | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
as hip or knee replacements. In such patients, the rate of venographically detected VTE may be as high as 40 to 60% without prophylaxis. After hemostasis is achieved postoperatively, DVT prophylaxis should be initiated in all patients with an important risk of DVT. In some situations, prophylaxis may extend beyond discharge. A high index of suspicion must be maintained if any symptoms of VTE occur.
Vascular thrombosis (venous/arterial/small vessel) in any tissue/organ. Superficial venous thrombosis is not included in clinical criteria.
Pregnancy morbidity
Lupus anticoagulant present in plasma/serum >2 occasions at least 12-weeks apart
Anticardiolipin antibodies (IgG and IgM) in plasma/serum > 2 occasions at least 12-weeks apart
Anti-β2 glycoprotein 1 antibodies (IgG and IgM) in plasma/serum > 2 occasions at least 12-weeks apart
thrombotic event, not all are strongly linked to an increased risk of recurrent events. Thus, caution must be used while interpreting the results of these tests, and widespread screening in all patients with VTE is not recommended.
First presentation at a young age
Strong family history of VTE
Recurrent idiopathic VTE and VTE in unusual sites (portal, splenic, mesenteric, or cerebral vein thromboses)
Extensive VTE
is not specific for venous thrombosis with the specificity reduced in patients with cancer, pregnant women, and hospitalized and elderly patients. Techniques that detect d-dimer include enzyme-linked immunosorbent assays, latex agglutination and immunoturbidimetric tests, and whole blood agglutination methods. Specific assays that have been validated for use in VTE diagnosis include (i) SimpliRED d-dimer (Agen Biochemical Ltd., Brisbane, Australia), (ii) Vidas d-dimer (bioMérieux, France), (iii) MDA d-dimer (bioMérieux, Durham, NC), and (iv) Tiniquant d-dimer (Roche Diagnostics, The Netherlands). Results of each test cannot be generalized to other methods of d-dimer quantification due to the wide variety of assay techniques. Generally, though, all are characterized by an intermediate to high sensitivity coupled with an intermediate to low specificity. This allows a negative d-dimer, in combination with a low clinical pretest probability, to effectively rule out acute VTE (less than 1% chance that a patient has VTE). Positive d-dimer results, however, do not equate to a diagnosis of VTE, and further testing is required.
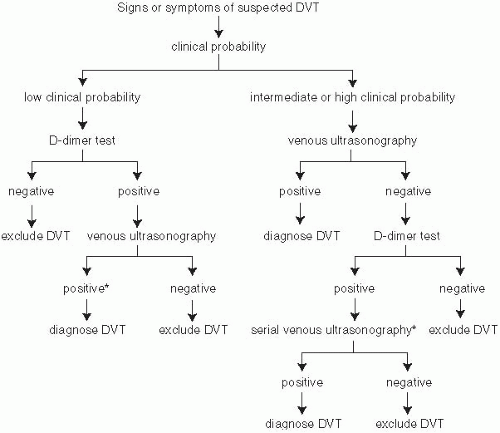 FIGURE 21-1. Diagnostic algorithm for deep vein thrombosis. (From Hirsh J, Lee AYY. How we diagnose and treat deep vein thrombosis. Blood 2002;99:3102-3110.) |
promising. d-dimer testing may help to make decisions on whether to stop or discontinue anticoagulation therapy. Troponin and BNP measurements may help to stratify patients with PE for their risk, with several studies
showing an association between elevation and short-term risk for dying and recurrent PE. Conversely, the absence of elevation in these parameters is a favorable prognostic factor, with limited studies suggesting an almost 100% negative predictive value for complications if plasma BNP levels are normal. The use of troponin with RV functional abnormalities may also be helpful with the absence of right ventricular dysfunction and a normal troponin level identifying patients who are eligible for early discharge or even outpatient treatment.
TABLE 21.2 CLINICAL PREDICTION RULE FOR PREDICTING DVT | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||
TABLE 21.3 CLINICAL PREDICTION RULE FOR PREDICTING PE | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree