Fig. 40.1
Physiology of vascular smooth muscle contraction and relaxation and mechanism of action of various vasodilators. This figure shows a simplified version of vascular smooth muscle physiology. Activation of MLCK promotes contraction, and activation of MLCP promotes relaxation. Vasodilators act via different signaling pathways as shown in the figure causing inactivation of MLCK or activation of MLCP resulting in vasodilation. Abbreviations: Ca ++ calcium ions, CM calmodium, MLCK mysoin light chain kinase, MLCP mysoin light chain phosphate, NO nitric oxide, cGMP cyclic gunalyl monophosphate, cAMP cyclic adenosine monophosphate, Gs stimulatory regulative G protein, Gi inhibitory regulative G protein, IP3 inositol triphosphate, Gq class of G protein acting via IP3 pathway
1.
Reduction in the amount of available intracellular calcium ions:
This can be achieved by blocking the voltage-gated L-type calcium channels or by reducing the release of calcium from sarcoplasmic reticulum. Calcium channel blockers such as nicardipine and clevidipine act via this pathway (Chap. 37).
2.
Activation of signal transducing pathway via cyclic guanosine monophosphate (cGMP):
The vascular endothelium, the innermost layer of blood vessels, secretes substances such as nitric oxide and endothelins that act directly on the vascular smooth muscle (Chap. 31). Nitric oxide, produced during the conversion of L arginine to L citrulline, activates guanylyl cyclase, which catalyzes the conversion of guanosine triphosphate (GTP) to cGMP. This results in activation of protein kinase C (PKC) which increases the phosphatase activity of myosin light-chain phosphatase (MLCP). MLCP in turn dephosphorylates the myosin light chains causing relaxation of the vascular smooth muscle. PKC also phosphorylates and inhibits Rho kinase and promotes vasodilation. Sodium nitroprusside and nitroglycerin are the two most commonly used drugs in hypertensive emergencies that act by the production of nitric oxide.
3.
Activation of G protein-coupled signal transduction:
The adrenergic receptors regulate vasodilation via the G protein-coupled signal transducers. Stimulatory regulative G proteins (Gs) are coupled to beta 2 agonists and dopamine D1 receptors in the vascular smooth muscle. These proteins stimulate adenylyl cyclase, which catalyzes the formation of cyclic adenosine monophosphate (cAMP) (Chap. 5). This inhibits MLCK causing deformation of the cross bridges between myosin and actin filaments, resulting in relaxation.
The inhibitory regulative G proteins (Gi) in vascular smooth muscles are coupled to alpha 2 adrenoreceptors. The activation of these receptors causes a reduction in cAMP, activation of MLCK, and hence vasoconstriction. Peripheral alpha antagonists such as phentolamine and labetalol act by blocking this pathway.
The Gq proteins are coupled to alpha 1 adrenoreceptors, AT1 receptors (bind to angiotensin II), and V1 receptors (bind to vasopressin). These Gq proteins are linked to the inositol triphosphate pathway (IP3) which stimulates the release of calcium from sarcoplasmic reticulum causing vasoconstriction. Alpha-1 antagonists and angiotensin-converting-enzyme inhibitors act as vasodilators by inhibiting this pathway (Chap. 36).
40.3 Pharmacology of Individual Drug Classes
Depending on the site and mechanism of action, vasodilators used in hypertensive emergencies are divided into the following classes:
1.
Directly acting vasodilators – act via nitric oxide pathway
2.
Calcium channel blockers
3.
Beta adrenergic blockers
4.
Alpha adrenergic blockers
5.
Dopamine agonists
The chemical structures of all the discussed drugs in this chapter are provided in Fig. 40.2 and are listed with their pharmacokinetic properties in Table 40.1.
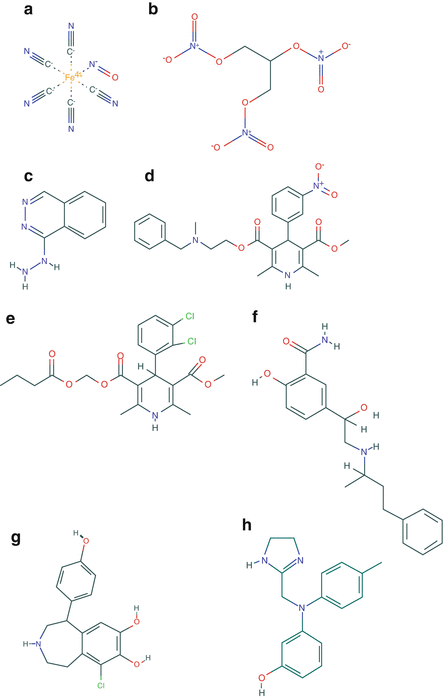

Fig. 40.2
Chemical structures of all the described vasodilators. (a) Sodium nitroprusside, Pubchem CID 11963622. (b) Nitroglycerin, Pubchem CID 4510. (c) Hydralazine, Pubchem CID 3637. (d) Nicardipine, Pubchem 4474. (e) Clevidipine, Pubchem CID 153994. (f) Labetalol, Pubchem CID 3869. (g) Fenoldopam, Pubchem CID 3341 h). (h) Phentolamine, Pubchem CID 5775 (source: Pubchem, https://pubchem.ncbi.nlm.nih.gov/; Accessed on August 22nd, 2014)
Table 40.1
Vasodilators commonly used in the management of hypertensive emergency
Drug | Mechanism of action | Dose | Onset | Half-life | Clinical situation | Precautions/contraindications |
|---|---|---|---|---|---|---|
Sodium nitroprusside | Arterial vasodilator via c GMP pathway | 0.25–10 μg/kg/min | 1–2 min | Nitroprusside, circulatory 2 min; thiocyanate 2 days | All clinical situations of hypertensive emergencies. Caution in neurological emergencies as it decreases cerebral perfusion and in ACS can cause coronary steal phenomenon | Elevated intracranial pressure. Renal and hepatic impairment |
Nitroglycerin | Venous vasodilator via cGMP pathway | 5–400 μg/min | 2–5 min | 1–4 min | Commonly used in acute coronary syndrome and decompensated heart failure | Concomitant use of phosphodiesterase inhibitors, inferior ST segment elevation myocardial infarction |
Nicardipine | Dihydropyridine calcium channel blocker | 5–15 mg/h | 10 min | 2–4 h | Postoperative hypertension, neurological emergencies | Severe aortic stenosis, advanced heart failure |
Clevidipine | Dihydropyridine calcium channel blocker | 1–21 mg/h | 2–4 min | Biphasic | Potentially useful in most hypertensive emergencies; extensively studied in post-cardiac surgery patients | Allergy to soy and egg products, advanced heart failure, severe aortic stenosis |
Initial: 1 min (predominant) | ||||||
Terminal: 15 min | ||||||
Fenoldopam | Peripheral dopamine 1 receptor agonist | 0.03–1.6 μg/kg/min | 10 min | 5 min | Hypertensive emergencies complicated by renal failure | Sulfite allergies, glaucoma |
Labetalol | Combined alpha and beta antagonist | IV bolus: 20 mg over 2 min; infusion: 1–2 mg/min | 2–5 min | 6 h | Aortic dissection, neurological emergencies | Severe bradycardia, advanced decompensated heart failure |
Hydralazine | Direct arterial vasodilator | IV bolus: 10–20 mg | 5–20 min | 2–8 h | Preeclampsia and eclampsia | Dissecting aortic aneurysm |
Phentolamine | Alpha 1 and 2 blocker | IV bolus: 1–5 mg; infusion: 1–40 mg/h | 1–2 min | 19 min | Catecholamine excess states like pheochromocytoma | Acute coronary syndrome |
Sulfite allergies |
40.3.1 Direct-Acting Vasodilators
These drugs act directly on the vascular smooth muscle and cause vasodilation by increasing the endothelial concentration of nitric oxide.
40.3.1.1 Sodium Nitroprusside
Sodium nitroprusside is a complex anion with an octahedral iron center surrounded by five cyanide ligands and one linear nitric oxide ligand [8]. It interacts with oxyhemoglobin and immediately breaks down to release nitric oxide, cyanide, and methemoglobin. The active metabolite, nitric oxide, activates guanylyl cyclase in the vascular smooth muscle, which results in increased production of cGMP. The myosin light chains are dephosphorylated causing vascular smooth muscle relaxation and subsequent vasodilation [8]. Sodium nitroprusside is a nonselective vasodilator acting on both arterioles and venules (arterioles more than venules). Hence, it reduces both the afterload and preload and can sometimes cause reflex tachycardia due to the activation of baroreceptors. Compared to other antihypertensive agents, it has the quickest onset of action (<2 min) and the shortest half-life (2 min) [8]. Hence, it is easily titratable, and its effects are reversible immediately after stopping the infusion. It is excreted renally as thiocyanate, and hence it should be used with caution in patients with renal failure [8].
The use of sodium nitroprusside may be problematic for several reasons. Given the short duration of action, it can precipitously drop the blood pressure. The target blood pressure can be overshot compromising the tissue perfusion. Hence, it is recommended to use sodium nitroprusside only in the intensive care setting, preferably with continuous blood pressure monitoring with an intra-arterial line [9]. The drug is supplied in a lyophilized powder, which is reconstituted and then diluted before use. The resulting solution is sensitive to light and should be wrapped in an aluminum foil set or other opaque material to prevent exposure to light. The drug will remain stable for 24 h if protected from light [10]. A loss of the drug activity is reflected by a change in the solution color from light brown to dark brown, green, orange, or blue [11]. Prolonged exposure to large doses of the drug can result in cyanide toxicity [8]. Signs of cyanide toxicity include mental status changes, seizures, coma, tachyphylaxis, arrhythmias, and metabolic acidosis [8].
Sodium nitroprusside is a nonselective vasodilator and has its effect on most of the vascular beds [9]. In patients with hypertensive encephalopathy, it should be used with extreme caution, as the precipitous drop in the mean arterial blood pressure can result in cerebral hypoperfusion [12, 13]. Similarly in patients with coronary artery disease and ongoing ischemia, it is contraindicated to use sodium nitroprusside because it can potentially cause a substantial reduction in coronary blood flow by causing arteriolar vasodilation and intracoronary steal of blood flow from the ischemic areas [14]. Nitroprusside causes dilation of the resistance vessels. The resistance of the vasculature supplied by a coronary artery with significant stenosis is already very low due to the autoregulation in response to decreased pressure distal to the stenosis. So, these vessels are incapable of dilating further in response to vasodilators. Hence, when a vasodilator like sodium nitroprusside is used, resistance vessels of the non-stenosed artery are dilated, resulting in shunting or “stealing” of blood from the ischemic areas [14]. Use of sodium nitroprusside within 9 h after the onset of chest pain in patients with acute myocardial infarction and elevated left-sided filling pressures was associated with increased mortality [15]. In summary, though sodium nitroprusside can be used in management of most clinical situations of hypertensive emergency, the need for invasive hemodynamic monitoring, potential for cyanide toxicity, and reduction in cerebral blood flow do not make it the first line of agent in everyday clinical practice to treat hypertensive emergencies.
40.3.1.2 Nitroglycerin
Nitroglycerin or glyceryl trinitrate is an organic nitric acid ester formed by treating glycerol with nitric acid [16]. It also acts on the vascular smooth muscle by producing nitric oxide, however, by a mechanism different from that of sodium nitroprusside. It also has a relatively quick onset of action (2–5 min) and a short half-life (1–4 min) [16]. It undergoes extensive first-pass metabolism in the liver and is converted into inactive glycerol di- and mononitrate metabolites [16].
Nitroglycerin undergoes biotransformation into either its active or inactive metabolites [17]. Glutathione reductase and glutathione-S-transferase are the enzymes involved in degradation of nitroglycerin to inactive metabolites – inorganic nitrite and glycerol 1,3-dinitrate [17]. The mitochondrial enzyme aldehyde dehydrogenase results in bioactivation of nitroglycerin converting it into active metabolites – nitric oxide and glycerol 1,2-dinitrate [17]. The mechanism resulting in nitrate tolerance is complicated and not very well defined. Some of the proposed theories include nitroglycerin-induced activation and increased sensitivity to receptor-dependent vasoconstrictors such catecholamines and vasopressin. Nitroglycerin also increases the mitochondrial NADPH (nicotinamide adenine dinucleotide phosphatase) oxidase activity, producing superoxide and peroxynitrite free radicals which inhibit aldehyde dehydrogenase, resulting in reduced biotransformation of nitroglycerin [16, 17].
Nitroglycerin is more selectively a venodilator resulting in preload reduction and acts on arterioles only in very high doses [18]. It is also a coronary vasodilator and improves collateral blood flow and sub-endocardial perfusion resulting in reduced myocardial oxygen demand [18]. Because of its properties on the coronary arteries, it is the drug of choice in hypertensive emergencies associated with acute pulmonary edema or acute coronary syndromes. In volume-depleted patients, a preload reduction may lead to decreased cardiac output. Similar to sodium nitroprusside, it is a cerebral vasodilator and often causes headache as a side effect. It also should be used cautiously in patients with suspected raised intracranial pressure. Other common side effects include hypotension, dizziness, flushing, and nausea (Chap. 23).
40.3.1.3 Hydralazine
Hydralazine is a synthetic compound prepared by the action of hydrazine hydrate on 1-chloro or 1-phenoxyphthalazine [19]. It is a potent direct-acting arteriolar vasodilator. It causes vascular smooth muscle relaxation by various mechanisms – influx of potassium into the vascular smooth muscle and hyperpolarization, activation of guanyl cyclase and increase in cyclic GMP, inhibition of release of calcium ions from sarcoplasmic reticulum, and finally stimulation of nitric oxide formation by vascular endothelium [20]. The intravenous formulation has a latent onset of action between 5 and 20 min [21]. The half-life is longer compared to other directly acting vasodilators (2–8 h) [21]. It is hepatically acetylated and excreted by extensive first-pass effect [21]. Given the unpredictable antihypertensive effect that lasts for several hours and difficulty in titration, it is not recommended for use in the treatment of hypertensive emergencies. It is used in hypertensive crisis related to pregnancy as it increases the uterine blood flow and is not teratogenic [18].
40.3.2 Calcium Channel Blockers
Calcium channel blockers reduce the blood pressure by causing arterial vasodilation by blocking the calcium ion influx through L-type voltage-gated calcium channels in the vascular smooth muscle cells. The dihydropyridines are a class of calcium channel blockers that mainly act on the vascular smooth muscle, while the non-dihydropyridines act on the myocardium (Chap. 37).
40.3.2.1 Nicardipine
Nicardipine is a second-generation dihydropyridine class of calcium channel blocker. It blocks the L-type voltage-gated calcium channels in vascular smooth muscles [22] and reduces the calcium ion influx causing relaxation and dilation of the peripheral arteries. It has a rapid onset of action (5–15 min), and its effect lasts for about 4–6 h [23]. Nicardipine undergoes extensive first-pass metabolism in the liver. Sequential metabolism of N-benzyl side chain occurs resulting in production of pyridine analogue metabolites [23]. It also has a strong cerebral and coronary vasodilatory effect. Intravenous nicardipine has been shown to reduce cerebral ischemia. In the presence of an acidic pH from cerebral ischemic tissue, there is a high degree of protonated drug which allows for rapid accumulation leading to localized vasodilation [24]. It is often used in patients with hypertensive emergency and stroke after receiving tissue plasminogen activator (tPA) [25]. The most common side effects of the drug include headache, hypotension, nausea, vomiting, and tachycardia [24]. Short-acting calcium channel blockers should be used with caution. Oral or sublingual immediate-release nifedipine has been associated with increased mortality and acute myocardial infarction [26].
40.3.2.2 Clevidipine
Clevidipine is a third-generation dihydropyridine, which blocks the L-type calcium channels. The calcium ion influx through these channels is blocked during arterial smooth muscle depolarization causing vasodilation and reduction in blood pressure [27]. It reduces the afterload without a reduction in preload and, hence, does not cause reflex tachycardia. The product labeling includes a contraindication for use in patients with severe aortic stenosis as afterload reduction can be expected to result in reduced myocardial oxygen delivery [28]. Intravenous clevidipine has a rapid onset of action (1–5 min) and a short half-life (1 min) [27]. Clevidipine clearance does not depend on hepatic or renal function [27]. It is converted into an inactive metabolite by esterases in blood and extravascular tissue [27].
The safety and efficacy of clevidipine has been studied extensively in trials among patients presenting to the emergency room and in cardiac surgery patients [29–31]. In the phase III clinical trial, the Effect of Ultra-Short-Acting Clevidipine in the Treatment of Patients with Severe Hypertension (VELOCITY), among 126 patients who presented to the emergency room with hypertensive crises, 90 % of the patients treated with clevidipine reached target blood pressure within 30 min (median of 10.9 min) [30]. There were no hypotensive episodes related to the use of clevidipine in this study. Clevidipine has also been shown to be safe and efficacious in several randomized trials involving cardiac surgery patients with hypertensive crises in the postoperative period [30, 31].
Clevidipine is manufactured in a lipid emulsion and is contraindicated in patients with an allergy to soy or egg as well as in patients with defective lipid metabolism such as pathological hyperlipidemia, lipoid nephrosis, or acute pancreatitis if accompanied by hyperlipidemia [28]. Due to the lipid formulation of the product, no more than 1,000 ml or an average of 21 ml/h over 24 h is recommended [28]. Additionally, the product is discarded 12 h after the puncture of the stopper to prevent microbial growth [28].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


