and Ulrich Hoffmann1
(1)
Division of Vascular Medicine, Medical Clinic and Policlinic IV, Hospital of the Ludwig Maximilians-University Hospital, Pettenkoferstrasse 8a, Munich, 80336, Germany
Keywords
VasculitisVessel inflammationLarge vessel vasculitisGiant cell arteritisTakayasu arteritisBehçet’s diseaseIgG4Introduction
The systemic vasculitides constitute a heterogeneous group of disorders characterized by inflammation of the blood vessels. Within the wide-ranging clinical spectrum of vasculitides, critical limb ischemia (CLI) represents a rather rare disease manifestation. However, some forms of vasculitis predominantly involve the large- and medium-sized arteries. Because noninflammatory arterial diseases also typically involve these arteries and specialists in Vascular Medicine are regularly confronted with the clinical picture, this chapter focuses primarily on vasculitides of medium- and large-sized vessels. Among the variety of other vasculitides, those carrying the potential to cause CLI are discussed comprehensively. As CLI caused by vasculitis usually requires a distinct treatment approach, particular emphasis is placed on how to differentiate inflammatory from noninflammatory arterial disease.
Classification of Vasculitis
Infectious vasculitis is extremely rare and characterized by direct invasion of microbial pathogens into the vessel wall. Noninfectious vasculitides are much more likely to be encountered in Vascular Medicine practice. Due to inconsistencies in the nomenclature, e.g., the use of eponyms or historic disease terms not reflecting the current state of knowledge, classification of the noninfectious vasculitides has been unnecessarily confusing in the past.
In 1990, the American College of Rheumatology proposed classification criteria, aimed to enable clinical researchers to differentiate one vasculitis form from another in order to unify inclusion criteria for clinical trials [1]. In 1994, the Chapel Hill Consensus Conference reached a consensus on names and specific definitions for the most common forms of noninfectious vasculitis. This consensus was revised in 2012 and provides the basis for today’s nosology of vasculitis [2] (Table 26.1). The Chapel Hill nomenclature categorizes noninfectious vasculitides primarily according to type of vessels predominantly involved, but also integrates knowledge on etiology, pathophysiology, and clinical and pathological characteristics. However, one must be aware that, as a matter of principle, every type of vasculitis may affect vessels of any size [2].
Large vessel vasculitis (LVV) |
Takayasu arteritis (TAK) |
Giant cell arteritis (GCA) |
Medium vessel vasculitis (MVV) |
Polyarteritis nodosa (PAN) |
Kawasaki disease (KD) |
Small vessel vasculitis (SVV) |
Antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) |
Microscopic polyangiitis (MPA) |
Granulomatosis with polyangiitis (Wegener’s) (GPA) |
Eosinophilic granulomatosis with polyangiitis (Churg-Strauss) (EGPA) |
Immune complex SVV |
Anti-glomerular basement membrane (anti-GBM) disease |
Cryoglobulinemic vasculitis (CV) |
IgA vasculitis (Henoch-Schönlein) (IgAV) |
Hypocomplementemic urticarial vasculitis (HUV) (anti-C1q vasculitis) |
Variable vessel vasculitis (VVV) |
Behçet’s disease (BD) |
Cogan’s syndrome (CS) |
Single-organ vasculitis (SOV) |
Cutaneous leukocytoclastic angiitis |
Cutaneous arteritis |
Primary central nervous system vasculitis |
Isolated aortitis |
Others |
Vasculitis associated with systemic disease |
Lupus vasculitis |
Rheumatoid vasculitis |
Sarcoid vasculitis |
Others |
Vasculitis associated with probable etiology |
Hepatitis C virus-associated cryoglobulinemic vasculitis |
Hepatitis B virus-associated vasculitis |
Syphilis-associated aortitis |
Drug-associated immune complex vasculitis |
Drug-associated ANCA-associated vasculitis |
Cancer-associated vasculitis |
Others |
Of note, some obviously inflammatory vasculopathies are not included in the Chapel Hill nomenclature, with Buerger’s disease (see Chap. 21) being the most important of these from the vascular specialist’s point of view.
Large Vessel Vasculitis (LVV )
According to the Chapel Hill nomenclature, the primary vasculitides predominantly involving the large- and medium-sized vessels are giant cell arteritis (GCA) and Takayasu arteritis (TA) [2].
Epidemiology
Being the most common form of vasculitis in Europe and North American countries, GCA can be considered also the most common inflammatory arteriopathy resulting in ischemia of the upper or lower limbs. GCA almost exclusively affects individuals aged 50 years and older, with women 2–3 times more frequently affected than men. In populations of Caucasian descent, annual incidence rates of up to 20/100,000 people have been reported, peaking in the 70–79 years age group [3]. There is a strong association with polymyalgia rheumatica (PMR).
TA is a rare disease, with annual incidence rates of less than 1 per million population in the USA and Europe. Higher incidence rates are found in Asian countries. The age of onset is typically before 40 years, and women are affected in up to 90 % of cases [4].
Pathophysiology
Both GCA and TA are characterized by a granulomatous panarteritis of the aorta and its major branches, going along with a variable systemic inflammatory response. The histological pattern is indistinguishable between both disorders and characterized by a segmental cell infiltrate composed of T lymphocytes, macrophages, and multinucleated giant cells, fragmentation of the internal elastic lamina, and myointimal hyperplasia [5].
A definite trigger eliciting the deregulated interaction between the arterial wall and the immune system has not been established yet. The typical vessel tropism observed in LVV could be the result of stimulation of distinct profiles of toll-like receptors mediating activation of vascular dendritic cells in different arterial territories [6]. Activated vascular dendritic cells attract CD4 T lymphocytes and macrophages by secretion of certain chemokines. In early and untreated vasculitis, interferon-γ-producing TH1 T lymphocytes and interleukin-17-producing TH17 T lymphocytes are abundant, surrounded by activated macrophages and multinucleated giant cells in granulomas [7].
Interferon-γ mediates macrophage activation as well as proliferation and migration of vascular smooth muscle cells [5]. Macrophages have various effector functions which differ according to their localization within the inflamed vessel wall. Macrophages recruited to the expanding intimal layer further stimulate migration and proliferation of medial smooth muscle cells by producing growth factors such as platelet-derived growth factor. Vascular remodeling by means of myointimal hyperplasia results in luminal stenosis or occlusion and is the fundament of tissue ischemia in LVV.
Differentiation of interleukin-17-producing TH17 T lymphocytes is promoted by interleukin-6, a pleiotropic cytokine secreted by immune and vascular cells. The TH17 subpopulation can be effectively suppressed by corticosteroid treatment, whereas the TH1 subpopulation seems to persist in the arterial wall even under long-term corticosteroid treatment [7]. These findings have important clinical implications as they indicate that LVV is not a self-limiting disease and that structural vascular damage due to sustained arterial inflammation may occur in the long term despite immunosuppressive treatment .
Clinical Spectrum of GCA
Nowadays, GCA is recognized as a systemic vascular disease, with a variable disease pattern typically affecting the aorta and its major branches but also smaller-sized arteries such as the branches of the external carotid arteries (e.g., temporal and maxillary arteries) and ophthalmic arteries (e.g., posterior ciliary arteries) [8]. With increasing age, a continuous shift from extracranial large artery involvement to isolated cranial artery involvement can be seen, with a considerable overlap of both disease patterns in about 50 % of patients [9].
Best known are the cranial symptoms of the disease, comprising new-onset headache, jaw claudication due to ischemia of the mastication muscles, and tender and swollen superficial temporal arteries [3, 8]. Cranial GCA is frequently (in up to 20 % of cases) complicated by ischemic ocular complications , mainly AION, carrying a significant risk of uni- or bilateral persistent visual loss. Much less frequent is cerebral ischemia, mainly in the posterior circulation due to vertebral artery involvement. Forty to sixty percent of patients complain of symptoms of polymyalgia rheumatica, and about 40 % of patients suffer from systemic symptoms such as low-grade fever, fatigue, and weight loss [3].
Limb ischemia mainly occurs in early untreated disease but also has been reported to develop in known GCA during corticosteroid tapering [10, 11]. Applying modern noninvasive vascular imaging methods, involvement of the subclavian and axillary arteries has been observed in 25–75 % of patients (Fig. 26.1) [9, 12–14]. Obstructive lesions are typically localized in the axillary arteries, whereas the proximal subclavian arteries remain patent. Therefore, subclavian steal phenomenon is not a feature of extracranial GCA. Abundant collateralization via the subscapular and circumflex humeral arteries provides sufficient blood supply to the arms even in case of complete axillary occlusion (Fig. 26.2). The brachial arteries are rarely affected, but brachial artery involvement may increase the risk of hemodynamic decompensation due to impairment of the collateral inflow [10]. Bilateral involvement is a hallmark of upper extremity vasculitis in GCA [9, 12–14].
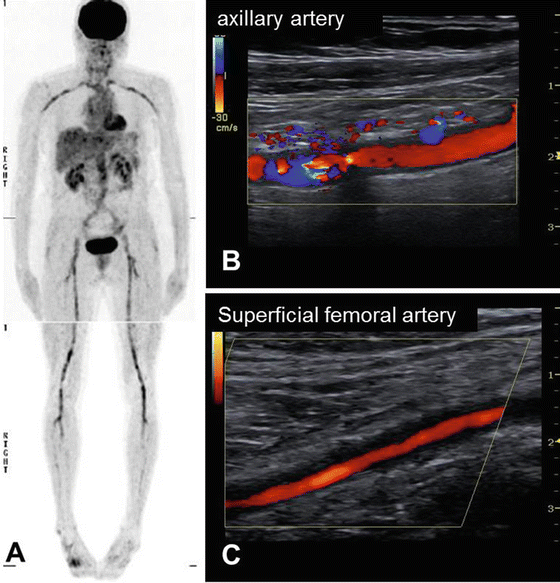


Fig. 26.1
Distribution of upper and lower extremity vasculitis in GCA, as visualized by PET (a) and CDS (b, c). PET 18F-fluorodeoxyglucose-positron emission tomography, CDS color duplex sonography

Fig. 26.2
Collateralization of axillary artery obstructions in GCA. MRA depicting bilateral axillary artery occlusions with abundant collateralization in a female patient with GCA (a). CDS revealing retrograde flow in the left subscapular artery, filling the distal left axillary artery behind left axillary artery occlusion secondary to GCA (b). MRA magnetic resonance angiography, CDS color duplex sonography
As a rule of thumb, vasculitis of the subclavian/arteries leads to luminal stenosis or occlusion in two thirds of cases and to symptomatic arm ischemia in only one third of cases [9]. In our cohort followed at a University Vascular Medicine Center, symptomatic upper limb ischemia was documented in every fifth patient suffering from GCA. The typical symptoms of upper limb ischemia are intermittent claudication and Raynaud’s phenomenon, with bilateral symptoms reported in 15–50 % of cases [9, 19]. Of note, classical cranial symptoms are absent in the majority of patients presenting with upper extremity ischemia as the leading disease manifestation [9]. Conversely, recognition of arm claudication in GCA may be impaired due to concomitant symptoms of polymyalgia rheumatica or ischemia of the brachial plexus.
Critical ischemia of the upper limbs with rest pain and/or digital necrosis seems to be essentially rare in GCA [9, 15]. Exceptional cases of digital necrosis due to upper limb ischemia in GCA requiring revascularization have been described in the literature [15]. We observed critical upper limb ischemia in a patient with bilateral occlusion of the axillary arteries expanding to the brachial arteries. Other, sporadic causes of upper limb ischemia in GCA include stent graft covering of the left subclavian artery’s origin during thoracic endovascular aneurysm repair and small vessel vasculitis of the digital arteries.
It is very likely that lower extremity vasculitis in GCA has been misinterpreted as arteriosclerotic disease in many cases before noninvasive vessel wall imaging was introduced [8]. Applying these noninvasive vascular imaging modalities such as color duplex sonography (CDS) and 18F-fluorodeoxyglucose-positron emission tomography (PET), rates of lower extremity arterial involvement between 25 and 50 % have been detected [12, 13, 16]. The disease process is bilateral in almost all cases, and the arterial segments predominantly affected are the femorocrural arteries (Fig. 26.3) [11, 12, 16]. The most important difference between upper and lower extremity vasculitis in GCA is that lower extremity vasculitis frequently results in extensive wall thickening of long arterial segments. Multisegmental disease involving also the deep femoral arteries significantly impairs the capability of the femorocrural axis to collateralize arterial obstructions. Preexisting arteriosclerosis is common in the lower limbs in the age group affected by GCA and may further contribute to hemodynamic impairment.


Fig. 26.3
Digital subtraction angiography showing the widespread character of lower extremity vasculitis in GCA with diffuse obstructions of the femorocrural arteries and impaired collateralization due to diffuse deep femoral artery involvement (a, b). Dissection of the proximal superficial femoral artery (b)
The rate of symptomatic lower extremity vasculitis in our cohort of patients with GCA was 15 % [16]. The more than twofold increased risk of peripheral arterial disease observed in patients with polymyalgia rheumatica also might be attributable to occult lower extremity vasculitis [17]. The typical clinical presentation is bilateral and rapidly progressive calf claudication, frequently but not necessarily accompanied by arm claudication, cranial symptoms, and/or systemic manifestations [16]. Lower extremity vasculitis may be the only disease manifestation in rare cases and has been shown to result in CLI with tissue loss in a significant proportion of patients with GCA (15–30 % in published case series) [11, 16]. In some of the reported cases, spontaneous superficial femoral artery dissection contributed to hemodynamic deterioration (Fig. 26.3) [8, 16].
Clinical Spectrum of TA
Early TA is characterize d by systemic symptoms, including fatigue, weight loss, night sweats, myalgia, carotidodynia, and low-grade fever, and therefore is referred to as “prepulseless phase” of TA. With advancing disease obstructions of the aortic branches occur, and progressing vascular symptoms dominate the clinical presentation (“pulseless phase”) [4].
The supraaortic branches are almost always affected, and arm claudication, pulse loss, or subclavian bruits are prominent features of advanced TA. Contrasting to GCA, stenoses and occlusions typically involve the proximal subclavian arteries. Therefore, the subclavian steal phenomenon is not uncommon. As carotid involvement also frequently leads to significant stenoses, some patients will experience symptomatic cerebral ischemia. Arterial hypertension reflects renal ischemia secondary to (bilateral) renal artery stenosis, and symptomatic coronary involvement occurs occasionally. Symptomatic lower extremity ischemia is a rather infrequent manifestation of the disease, in most cases resulting from aortoiliac obstruction (Fig. 26.4).


Fig. 26.4
MRA depicting long-segment tight stenosis of the left subclavian and carotid artery, occlusion of the left axillary artery, (a) and bilateral common iliac arteries (b) in patients with TA suffering from upper and lower extremity ischemia, respectively. MRA magnetic resonance angiography
Although many patients experience a significant time delay between symptom onset and diagnosis, CLI is quite uncommon due to excellent collateralization. By contrast, critical organ ischemia due to renal or coronary artery obstructions may occur .
Diagnosis of LVV
Assessment of arterial hemodynamics in limb ischemia with suspected underlying LVV follows the general principles of noninvasive vascular laboratory testing. Validated diagnostic criteria for the LVV are not existent. Moreover, it must be stressed that patients with extracranial GCA frequently do not meet the ACR classification criteria for cranial GCA [9]. Advanced noninvasive vascular imaging has dramatically improved the diagnosis of the LVV.
CDS can be considered the first-line imaging technique in suspected LVV [18]. CDS enables the investigator to accurately assess the arterial wall of the aortic branches for the presence of a hypoechogenic, circumferential, homogenous wall thickening as an almost pathognomonic sonographic sign of LVV (Fig. 26.1). Limited data available suggest that the circumferential, hypoechogenic wall thickening is highly specific for LVV, with a diagnostic accuracy comparable to that of PET imaging [12, 19]. However, this imaging modality is observer dependent, and the diagnostic accuracy may be hampered in the lower extremity arteries because of concomitant arteriosclerotic changes [16]. In every patient suspected to suffer from LVV, it is mandatory to screen the subclavian/axillary and common carotid arteries for the presence of vasculitis. In our experience, 75 % of patients with symptomatic lower extremity vasculitis also exhibited vasculitic wall thickening of the subclavian/axillary arteries [9]. The superficial temporal arteries should regularly be visualized in patients above the age of 50 suspected of suffering from GCA .
Cross-sectional imaging, i.e., PET combined with computed tomography (PET-CT) or magnetic resonance imaging (MRI), should be reserved for ambiguous cases and revascularization planning. Features of LVV include increased contrast enhancement (MRI, CT) and increased tracer uptake (PET-CT) of the thickened vessel wall. It is of outmost importance to realize that a pathological tracer uptake of the upper extremity arteries in PET/PET-CT is highly specific for upper extremity vasculitis, whereas in the lower extremities, the specificity is reduced due to the increased tracer uptake seen with coexistent arteriosclerosis [18].
Digital subtraction angiography provides no information on the vessel wall morphology and thus is only indicated prior to revascularization (Fig. 26.3).
Medical Treatment of LVV
Systemic corticosteroids with an initial dose of 1 mg prednisone equivalent/kg/day (maximum 60 mg/day) remain the cornerstone of treatment in both GCA and TA [3, 4, 8]. In analogy to the treatment of GCA complicated by ocular ischemia, high-dose intravenous pulse treatment (1000 mg methylprednisolone) seems to be justified also in patients presenting with CLI on the basis of LVV. However, the benefit of this aggressive treatment approach is not proven and must be balanced against the potential risk of infection (particularly in those patients with ischemic ulcers). Corticosteroid tapering needs to be done very carefully and is allowed only in the absence of clinical and laboratory disease activity. After 3 months of treatment, one should aim at a dose of 10–15 mg prednisone equivalent/day. A minimum treatment duration of up to 2 years is recommended [3, 8]. However, relapses are common, and patients with GCA and widespread extracranial involvement and patients with TA appear to be particularly prone for a steroid-dependent or steroid-refractory disease course. As a result, more than 80 % of patients experience corticosteroid side effects. Unfortunately, only modest treatment effects have been documented for the conventional steroid sparing agents in the treatment of GCA and TA. A meta-analysis of three small randomized trials investigating adjunctive methotrexate treatment in GCA revealed a small benefit in terms of reduction of the cumulative corticosteroid dose and the recurrence rate [20]. Limited evidence for a potential benefit of other immunosuppressants such as azathioprine, cyclophosphamide, and leflunomide comes from case series. Randomized controlled studies failed to document a significant effect of the TNFα blockers infliximab and adalimumab as first-line treatment of GCA [21, 22]. However, these studies are in contrast to the results of open-label studies, documenting substantial treatment effects of TNFα blockers in refractory disease courses of TA [23]. Recently, promising results have been reported with the interleukin-6 receptor antagonist tocilizumab for achieving disease remission [23]. Currently, a randomized study investigating the effects of tocilizumab as adjunctive treatment in GCA is underway.
Although convincing data are lacking, antiplatelet treatment (aspirin) is recommended in addition to immunosuppressive medication in both types of LVV [3, 4, 8]. Because of the combination of aspirin with corticosteroids, proton pump inhibitors should be added to the medication. A benefit of adjunctive statin treatment is not evident. Under corticosteroid treatment, medical prevention of osteoporosis according to current guidelines is mandatory .
Treatment of Limb Ischemia in LVV
The current knowledge on surgical and endovascular treatment of LVV mainly comes from cohort studies having retrospectively evaluated patients with TA. Data on revascularization attempts in GCA are even less convincing, and randomized controlled trials are not available.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


