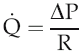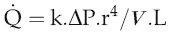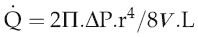Chapter 5 Vascular system
After reading this chapter, you should:
The volume flow of blood around the circulation per unit time (
) can be expressed in terms of the pressure gradient (ΔP) and the absolute resistance to flow of the vasculature (R):
Intravascular pressure at the downstream end of the vascular circuit where it drains into the heart is virtually the same as atmospheric pressure and can be regarded as 0 mmHg. The value of ΔP, therefore, depends primarily on the value of arterial blood pressure. In the last chapter we looked at the aspects of cardiac function that affect this and reviewed briefly the involvement of the peripheral vasculature. We need now to examine in more detail the properties of blood vessels and how these contribute to resistance to flow.
PHYSICAL FACTORS AFFECTING PERIPHERAL RESISTANCE
Vessel radius
The dependence of resistance on absolute vessel size also indicates that the major component of total peripheral resistance must be localized to the smaller precapillary vessels (the microcirculation) rather than the large distributing arteries. Not only are these microcirculatory vessels smaller in radius but they also branch repeatedly every mm or so. Although total cross-sectional area increases with each branching, total surface area increases even more, so that resistance rises rapidly along quite a short distance.
Blood viscosity
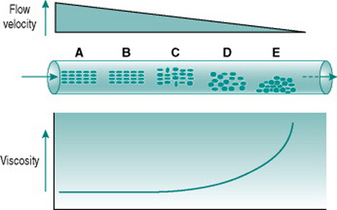
Figure 5.1 illustrates the processes that underlie anomalous viscosity in the bloodstream. When the blood is flowing relatively fast (A–B), the cellular components travel as a core in the centre of the vessel, surrounded by a cell-free layer of plasma. The cells are oriented so that they travel edge-on, producing minimal friction between the cell layers and between cells and plasma. The viscosity of the blood in this situation is around 50% greater than that of plasma alone. If the flow velocity falls sufficiently then the orientation of the suspension becomes less organized, with some cells starting to rotate and collide with adjacent cells. This process absorbs some of the energy creating the pressure gradient and so viscosity rises (C–D). If flow rate falls even further, the cellular constituents fall out of suspension and form an aggregate on the gravitationally lowest surface of the vessel. Because of the mass of the aggregate, a substantial amount of energy is required to lift the cells back into suspension again, reflected in a very high viscosity (E).
The dependence of blood viscosity on the cellular components has several implications. First, alteration of the haematocrit will change the size of the cell-rich core relative to surrounding plasma. Therefore, increased haematocrit will proportionately elevate viscosity. This can impose a substantial extra cardiac workload particularly during exercise. Thus, ‘blood doping’ with erythropoietin, although it is likely to improve maximal exercise performance by enhancing oxygen delivery to muscles, also carries a significant risk of damage to the heart. A second consequence of anomalous viscosity is that very low rates of blood flow can result in cells falling out of suspension. If the cell aggregates remain unsuspended for more than a few minutes, they begin to stick together and, in small vessels, may completely obstruct the lumen. This situation is most likely to occur in the postcapillary venules and will be discussed in Chapter 10 in relation to hypotensive states associated with prolonged exercise.
Blood flow through systemic capillaries is rather different to that through either precapillary or postcapillary vessels. All these other vessels have diameters greater than that of blood cells, whereas the typical capillary diameter of around 6 μm is marginally less than that of an erythrocytes (8 μm). In consequence, blood cells have to be partially folded in order to pass along the capillary. This process is important in that it ensures the closest contact possible between erythrocyte and endothelial membranes and so minimizes the distance for gas diffusion between blood and tissue. However, in theory it should also produce very high frictional forces between blood cells and the capillary wall, greatly increasing local viscosity and impeding the efficiency of capillary perfusion. To avoid such a disadvantageous situation, the endothelium of capillaries secretes a lubricant mucopolysaccharide that virtually eliminates frictional interaction with the blood cells and results in local viscosity that is almost as low as that of cell-free plasma.
Poiseuille’s law
The equation relating flow, pressure and resistance (see p. 45) can be rewritten by substitution of vessel radius (r) and length (L) and blood viscosity (V) for resistance, and adding a factor for numerical accuracy (k), thus:
The practical importance of Poiseuille’s law is that it describes the main factors affecting the relationship between flow and pressure. It does not of course take account of homeostatic feedback so does not necessarily predict the end result of manipulating these factors in the whole body. For example, if blood pressure were elevated by a sudden increase in blood volume, there would normally be baroreflex compensation that would lower peripheral resistance and return the pressure to its initial level (see Chapter 7, p. 81). A second difference from the events in the intact circulation is that Poiseuille’s law assumes that the vasculature is a system of rigid tubes. As soon as we deal with tubes that might distend in response to internal pressure, then obviously the association between pressure and flow becomes different.
RESISTANCE TO FLOW OF DISTENSIBLE VESSELS
Roles of connective tissue and muscle in determining distensibility
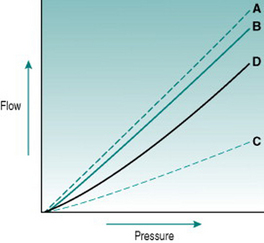
In rigid tubes, there is a linear relationship between the pressure gradient and the resulting volume flow, presuming that resistance remains constant (Fig 5.2A). In distensible tubes, by contrast, increased internal pressure will cause some increase in tube volume, so that the rate of volume flow rise with pressure will be lower. In blood vessels, distensibility is conferred by the presence of elastin in the vessel wall, while the presence of collagen confers rigidity (Fig. 5.2B–D). The property of distensibility is usually referred to as compliance.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree