Molecule
Molecular weight (kDa)
Normal levels
Concentration in advanced CKD
Potential contribution to vascular injury
Adiponectin
30
<11 mg/L
>15 mg/L
Low levels associated with vascular injury, high concentrations may reflect weight loss [46]
CRP
115
1–3 mg/L
>3 mg/L
Interleukin-6
24.5
<4 ng/L
>10 ng/L
Leptin
16
<10 μg/L
>100 μg/L
Controversial, possibly sympathetic nerve activity, endothelial dysfunction, platelet aggregation and vascular smooth muscle cell proliferation [46]
Pentraxin-3
40.2
<5 ng/mL
>10 ng/mL
Endothelial dysfunction [50]
Resistin
12.5
<15 μg/L
60 μg/L
Controversial, sympathetic nerve activity, endothelial dysfunction, platelet aggregation and vascular smooth muscle cell proliferation [46]
SAA
12
2–5 mg/L
>5 mg/L
Activation of monocytes, reversal of the anti-inflammatory properties of HDL [53]
Visfatin
55
1–2 ng/ml
10 ng/ml
Controversial, possibly endothelial damage, inflammation, plaque destabilization [46]
C-Reactive Protein (CRP)
In the clinical setting, C-reactive protein (CRP) is the molecule that is most often used as a marker to monitor inflammation. Synthesis of CRP in the liver occurs during a wide range of acute and chronic inflammatory conditions, but also in malignancies or tissue injuries. With its 19-h half-life, CRP is easy to detect in the circulation. The average CRP level ranges from 1 to 3 mg/l in the general adult population [20] while it is typically twice as high in dialysis patients [21, 22]. Similarly, patients with CKD not yet on dialysis display CRP concentrations higher than those observed in the general population and the probability of having an increased CRP rises as GFR decreases [9]. The exact mechanisms that lead to increase in CRP are still not clear. However, apart from well-established clinical causes such as intercurrent illnesses, especially infections, the most probable factors responsible include: retention of circulating cytokines, advanced glycation end-products (AGEs), pro-oxidants, sympathetic overactivity, and the impact of the dialysis procedure itself [23–25].
Studies in vitro suggest that CRP is involved in vascular processes contributing to atherosclerosis. CRP is found in lipid-laden plaques [26] where its ability to facilitate monocyte adhesion has been documented [27]. Moreover, CRP inhibits endothelial nitric oxide synthase, and, hence, impairs vasoreactivity [28]. The concept that inflammation in general, and/or specifically the CRP may contribute to vascular risk was further strengthened by the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) [29]. In this trial, decrease in hsCRP following rosuvastatin administration was associated with a substantially lower cardiovascular risk, even though cholesterol concentration was normal in the studied subjects.
However, a growing body of evidence seems to negate a causative role of CRP in vascular pathology [30]. Experimental animals overexpressing CRP do not show features of accelerated atherosclerosis [31, 32]. Similarly, injections of large doses of CRP have no or minimal effect on atherosclerosis progression [33]. In the general population, large-scale Mendelian randomization studies have demonstrated that even marked elevations in CRP concentrations do not increase the risk of cardiovascular disease (CVD) [34, 35]. Similarly, there was no association between CRP haplotypes and cardiovascular risk in dialysis patients [36]. On the basis of these and other studies, CRP is currently regarded as a risk marker but not a risk factor for CVD.
Interleukin-6 (IL-6)
Intereleukin-6 (IL-6) is a 24.5 kDa molecule that promotes the activation and proliferation of lymphocytes, differentiation of B cells, leukocyte recruitment, and the induction of the acute phase protein response in the liver [23]. The concentration of IL-6, one of the most potent drivers of CRP production, is typically elevated in CKD [37]. The mechanisms for IL-6 increase in the course of CKD are far from being clarified but the most probable are similar to the ones responsible for CRP elevation described above. The concentration of IL-6 has been shown to independently predict cardiovascular complications and mortality both in the general population [38] and in CKD patients [39, 40]. Several mechanisms by which IL-6 could influence the vascular system have been put forward such as an increase in platelet count, impaired insulin sensitivity, increased fibrinogen synthesis, and release of adhesion molecules in the endothelium [41]. It has been postulated that the potential role of Chlamydia pneumonia on atherosclerosis progression is mediated by increased IL-6 synthesis [42]. Indeed, CKD patients in whom atherosclerosis progresses show increased prevalence of anti-chlamydia antibodies, and, at the same time, enhanced IL-6 concentration, which independently predicts atherosclerosis progression (Fig. 6.1) [43]. A direct role for IL-6 in vascular complications is further strengthened by Mendelian randomization studies showing that functional variants of IL-6 gene affect the risk of CVD, both in the general population [44] and in CKD [45]. These results support a causative role of IL-6 in atherosclerosis progression and suggest it might be a target for future interventional studies.
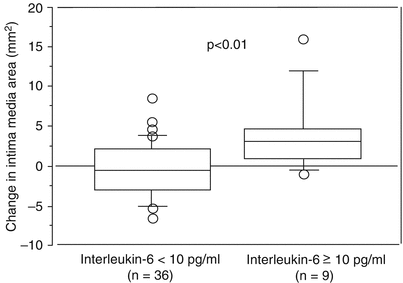

Fig. 6.1
Box plot showing a significant difference in changes of the calculated carotid artery area over 12 months between nine patients with IL-6 levels >10 pg/mL and 36 patients with IL-6 levels <10 pg/mL before start of dialysis treatment [43] (Reprinted from Yao et al. [19] with permission from Elsevier)
Adipokines
Adipokines are a group of bioactive proteins, such as leptin, adiponectin and resistin that are synthesized and released by adipocytes. They are believed to mediate the well-established association between obesity and increased cardiovascular risk. Their concentration in CKD it typically increased, possibly due, at least in part, to impaired renal clearance [46]. Experimental studies have documented the presence of adipokine receptors in atherosclerotic plaques. Moreover, their role in sympathetic nerve activity, endothelial dysfunction, platelet aggregation and vascular smooth muscle cell proliferation has been postulated [46]. As clinical studies evaluating the abovementioned associations have brought conflicting results [46] more studies are needed to establish whether adipokines could be considered as true risk factors for CVD.
Other Inflammatory Markers/Mediators
Pentraxin 3
Pentraxin 3 (PTX3) is a multifunctional soluble receptor modulating the immune-inflammatory response. It belongs to the same superfamily of acute-phase reactants as CRP and serum amyloid A. Concentrations of PTX3 have been found to be elevated in CKD, and to independently predict cardiovascular complications, both in dialysis and pre-dialysis patients [47, 48]. Elevated PTX3 may reflect endothelial dysfunction and be involved in adipose tissue-orchestrated mechanisms that modify inflammation and vascular complications in CKD.
Soluble TNF-Like Weak Inducer of Apoptosis (sTWEAK)
Soluble TNF-like weak inducer of apoptosis (sTWEAK) is a member of the tumor necrosis factor (TNF) superfamily. It is a glycoprotein with a molecular weight of 18 kD, which concentration tends to decrease in inflammatory conditions [49, 50]. Its levels have been shown to directly correlate with GFR, i.e. the lowest sTWEAK concentration has been reported in ESRD patients [51]. In this context, it is of interest that low sTWEAK has been shown to independently predict endothelial dysfunction in CKD subjects [52]. The mechanisms are still poorly understood, as for now it is difficult to discern whether sTWEAK has some protective role in endothelial functionality or merely is a marker of endothelial dysfunction and risk for cardiovascular complications.
Serum Amyloid A (SAA)
Serum amyloid A (SAA) is a member of a family of apolipoproteins associated with high-density lipoproteins (HDL) in plasma. Since uremia impairs the atheroprotective properties of HDL, the role of this acute-phase protein has been evaluated. Indeed, in experimental studies, SAA induces inflammatory reactions in human monocytes and may reverse the anti-inflammatory properties of HDL [53]. As SAA has failed to independently predict mortality in HD patients [54], its exact role in promoting atherogenesis in CKD remains to be established.
Other Inflammatory Markers
Obviously, the abovementioned inflammatory markers do not fulfill the whole list of molecules and conditions that are associated with inflammatory processes and that are dysregulated in uremia. One of the most important abnormalities involving both inflammation and vascular injury in CKD include the disordered calcium-phosphate balance, where calcium-phosphate deposits induce inflammatory response, and at the same time lead to vascular calcification, one of the most potent risk factors for cardiovascular mortality in CKD [55]. Recently, calciprotein particles (CPPS) have emerged as a possible novel link between calcium-phosphate disorders, inflammation and vascular calcification [56].
Anemia in CKD, primarily caused by deficiencies in erythropoietin and iron, is exacerbated by inflammation, and may contribute to CVD. Metabolic acidosis, another inherent feature of CKD, can be aggravated by inflammation, and contributes to atherogenesis through enhanced protein catabolism, insulin resistance and bone resorption. The limited size of this chapter does not allow for a thorough description of these, and other, important risk factors.
Oxidative Stress
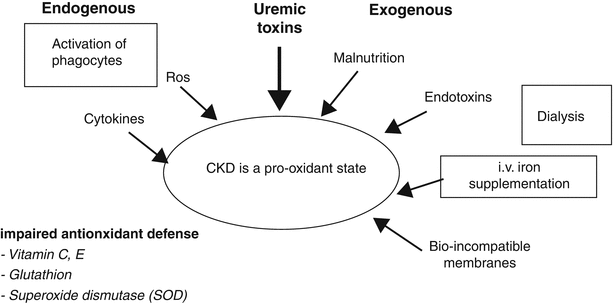
Oxidative stress can be defined as an imbalance between the generation of free radicals and the anti-oxidative capacity of surrounding tissues. Oxidative stress results in oxidation of macromolecules, including proteins, lipids, and carbohydrates, and is a widely acknowledged contributor to vascular disease in the general population. It is also an important feature of CKD, as increased levels of oxidative stress markers have been constantly demonstrated in uremic plasma [57]. Actually, oxidative stress starts early with renal function decline, and increased levels of oxidative markers have been documented already in CKD stage 3 [58]. Low-density lipoproteins (LDL) tend to be smaller and more dense in the course of CKD, and as such are more prone to oxidative modifications [59]. Indeed, numerous studies have shown that CKD, both at early stages and when dialysis-dependent, is associated with lipid and lipoprotein peroxidation [57]. Similarly, oxidatively modified plasma proteins and amino acids have also been documented in renal patients [57]. The exact mechanisms for oxidative stress in CKD are still poorly understood but are definitely associated with inflammation and PEW. As both these processes are prevalent in CKD, leading to hypoalbuminemia, it should be stated that the most important antioxidant capacity in plasma is provided by the thiol groups, which are largely located on the albumin molecule [57]. Therefore, patients with inflammation and/or PEW have a significantly diminished plasma antioxidant capacity. Other factors potentially responsible for increased oxidative stress in CKD have also been described (Fig. 6.2) [60]. Moreover, the uremic toxemia per se is postulated to augment oxidative stress, and, on the other hand, oxidative modifications of retained uremic solutes may potentiate their pathogenicity [57, 61]. As an example, recent evidence shows that HDL-cholesterol (regarded as anti-atherogenic) is transformed by symmetric dimethylarginine in the uremic milieu and paradoxically induces endothelial dysfunction via activation of toll-like receptor-2 [62]. In the clinical setting, few studies in CKD patients demonstrate associations between the intensity of oxidative stress and cardiovascular outcome [7, 63]. The impact of oxidative stress on hypertension and anemia, acknowledged cardiovascular risk factors, has also been postulated in CKD [64, 65]. Moreover, functional polymorphisms in genes encoding proteins involved in the antioxidant capacity have been shown to be associated with atherosclerosis, pointing to a casual role of oxidative stress in vascular complications in CKD [66, 67].
Therapeutic Possibilities of Anti-inflammatory and Anti-oxidative Treatment in CKD
The close relationships between inflammation, oxidative stress and atherosclerosis have led to studies evaluating drugs that would target inflammation and oxidative stress to diminish the cardiovascular risk. The current strategies are described below and summarized in Table 6.2. However, since inflammation and oxidative stress in CKD are driven by numerous factors, it has to be stated that we may never find a single silver bullet to tame these disorders. Instead, we may need a holistic approach involving among others: adequate dialysis prescription, use of biocompatible membranes and solutions, avoidance of central dialysis catheters, exercise implementation, adequate nutrition, efficient treatment of co-morbidities, and perhaps, on top of that, targeted anti-inflammatory and anti-oxidative medications.
Table 6.2
Therapeutic possibilities of anti-inflammatory and anti-oxidative treatment in CKD
Intervention | CKD population | Results |
|---|---|---|
Anti-cytokine therapies | ||
Etanercept | HD | Increase in albumin and prealbumin concentration [68] |
IL-1 receptor antagonist | HD | Reduction in CRP and IL-6 levels, increase in pre-albumin concentration [69] |
Anti-oxidative therapies | ||
Gamma-tocopherol | HD | Decrease in inflammatory markers concentration [76] |
Docosahexaenoic acid | HD | Decrease in inflammatory markers concentration [76] |
N-acetylcysteine | PD | Decrease in inflammatory markers concentration [77] |
Bardoxolone | Pre-dialysis | |
Nonspecific immunomodulatory approach | ||
Statins | Pre-dialysis | Reduction in CRP, and in mortality risk [83] |
Cholecalciferol | HD | |
Sevelamer | HD | Reduction in CRP and increase in getuin-A, improvement of endothelial function [92] |
Anti-cytokine Therapies
Given the potential of inflammatory molecules to directly modify the vascular risk, as described above, the hypothesis that by decreasing concentrations of these inflammatory markers one could improve the cardiovascular outcome is solid. However, only a few studies have tested anti-cytokine drugs in CKD patients. Administration of the TNF-receptor antagonist etanercept improved albumin and pre-albumin concentrations in HD patients [68]. In a similar cohort, treatment with a recombinant human IL-1 receptor antagonist resulted in a marked reduction in CRP and IL-6 levels as well as an increase in pre-albumin concentration [69]. Pentoxifylline is another drug with interesting anti-inflammatory effects, as it has been shown to improve protein breakdown along with an incremental anabolic effect [70, 71]. In the general population we are now witnessing an outbreak of studies focusing on targeted inhibition of IL-1, IL-6, phospholipase A2, CCR-2, and leukotrienes [72, 73]. Whether these therapies prove useful also in CKD patients, remains to be determined.
Anti-oxidative Therapies
Although, as described above, oxidative stress is enhanced in CKD, studies on anti-oxidative therapies show conflicting results. While some demonstrate no effect of treatment with tocopherols and alpha-lipoic acid in CKD stage 3–4 patients [74], nor in HD subjects [75], others show that supplementation with gamma-tocopherol, docosahexaenoic acid, or N-acetylcysteine has the potential to decrease IL-6 levels [76, 77]. Clearly, larger prospective trials are needed to verify these findings and to establish their impact on surrogate markers of vascular function and outcome.
The Nrf2 transcription system, which regulates genes involved in antioxidant and cytoprotective responses, may be an attractive target for intervention studies. Bardoxolone methyl, which acts by inhibiting pro-inflammatory transcription factors (such as NF-kB and STAT3), has shown promising results in CKD as it, in a small study, did slow down the progression of the CKD [78, 79]. However, a subsequent larger trial on bardoxolone was terminated prematurely due to an increased risk of cardiovascular events [80]. The reason for this unexpected result remains obscure, but potential hypotheses include: fluid retention, increased afterload, higher heart rate, and direct toxic effects. Since bardoxolone methyl activates a central transcription factor peroxisome proliferator–activated receptor γ, other, as yet unknown, mechanisms leading to volume retention and/or heart failure are also possible [81]. As various nutritional interventions may have weaker, but still meaningful, stimulatory effects on Nrf2, studies to evaluate if nutritional interventions may have an impact on inflammation and oxidative stress should be conducted [82].
Nonspecific Immunomodulatory Approach
In a secondary analysis of the JUPITER, rosuvastatin has been shown to reduce cardiovascular events and all-cause mortality among patients with moderate CKD and elevated CRP level [83]. In dialysis patients, the effect of statins is more controversial. While the SHARP (Study of Heart and Renal Protection) trial has demonstrated cardiovascular benefits of combined therapy with ezetimibe and simvastatin [84], other trials have shown no effect of statins on cardiovascular outcome, despite some impact on inflammatory markers [85, 86].
Vitamin D appears to play a special role in delaying atherosclerosis progression. Vitamin D receptors are present in all cells implicated in atherosclerosis, including endothelial cells, vascular smooth muscle cells, and immune cells. Experimental studies have revealed that vitamin D is involved in nitric oxide production and modulation of the vascular tone [87]. It significantly represses gene expression of prostaglandins, thromboxane A2, and of intercellular adhesion molecule-1, platelet endothelial cell adhesion molecule-1, and IL-6 in endothelial cells [87]. Following some promising results of in vitro studies, the anti-inflammatory potential of cholecalciferol supplementation has been evaluated. Indeed, cholecalciferol therapy was found to reduce circulating levels of inflammatory cytokines, including IL-8, IL-6, and TNF in HD patients with vitamin D deficiency [88, 89], as well as left ventricular mass index [90]. Moreover, administration of paricalcitol, a selective activator of the vitamin D receptor, was associated with a significant reduction in serum concentrations of CRP, TNF and IL-6, as well as a significant decrease in the mRNA expression levels of TNF and IL-6 in uremic peripheral blood mononucleated cells [91].
Sevelamer, probably through its lipopolysaccharide (LPS) binding potential, has been associated with a reduction in CRP and endotoxin levels [92] as well as higher fetuin-A levels [93], accompanied by an amelioration of endothelial dysfunction. Finally, it should be mentioned that several non-pharmacological interventions, such as short daily dialysis, exercise training, modification of nutrition (such as plant food, free fatty acids, probiotics etc.) may have impact on the smoldering uremic inflammation [94].
Conclusions
The risk of cardiovascular complications in CKD exceeds the one observed in the general population multifold. Current studies demonstrate that along with the traditional risk factors for CVD, inflammation and oxidative stress play a significant role in promoting atherosclerosis and vascular events. Both are highly prevalent in the course of CKD and seem not only to associate with cardiovascular risk but also to directly and causatively augment it. Although smaller studies show promising results of anti-inflammatory and anti-oxidative therapies in attenuating these complications, much larger trials are needed to verify their potential and clinical usefulness.
References
1.
Krol E, Rutkowski B, Czarniak P, Kraszewska E, Lizakowski S, Szubert R, et al. Early detection of chronic kidney disease: results of the PolNef study. Am J Nephrol. 2009;29(3):264–73.PubMedCentralPubMedCrossRef
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree