and Muzammil H. Musani2
(1)
Department of Radiology, Stony Brook University Hospital, 100 Nicolls road, Stony Brook, NY, USA
(2)
Department of Medicine and Radiology, Stony Brook University Hospital, Stony Brook, NY, USA
Arterial phase contrast studies, in particular when combined with multi-detector row CT capabilities, provide a highly useful diagnostic imaging tool for the evaluation of a multitude of vascular pathologies. In this chapter, we review in brief the epidemiology of several important angiographic diagnoses, and the clinical relevance of the pathophysiology underlying these clinical entities.
8.1 Aortic Aneurysm
The prevalence and incidence of thoracic aneurysms are difficult to ascertain accurately. As degenerative and atherosclerotic changes underlie most thoracic aortic aneurysms, the natural history is slowly progressive in the majority of cases. Genetic or inflammatory etiologies more commonly affect the thoracic rather than abdominal aorta. In two population studies [1, 2], annual incidence of thoracic aortic aneurysm was estimated to be 5.6 and 10.4 cases per 100,000 patient-years, likely an underestimation of true incidence as many such aneurysms are asymptomatic. Symptomatic thoracic aortic aneurysms are typically very large (5–6 cm or greater) and at increased risk for rupture.
Abdominal aortic aneurysms occur more commonly than the thoracic counterpart. Large-scale population screening studies demonstrate a prevalence of 4–8 % among elderly males, with increased risk of abdominal aortic aneurysm with advanced age, male gender, smoking, among other factors [3]. The majority of cases involve the infrarenal aorta, with approximately 5 % of cases involving the pararenal or suprarenal aorta. One retrospective analysis [4] of outpatients with abdominal aortic aneurysms found concomitant thoracic aortic aneurysm in approximately 25 % of subjects, with evidence of increased prevalence of comorbid thoracic aortic aneurysm in women; individually, thoracic and abdominal aneurysms have a male preponderance. Risk of rupture increases with aneurysm size and rate of growth, with larger aneurysm expanding at an increased rate [3–5].
8.2 Aortic Dissection
The primary event in acute aortic dissection is an intimal tear, leading to the characteristic intimal flap apparent on contrast-enhanced imaging studies. Propagation of the flap leads to extension of the dissection plane and creation of a false lumen, which can occlude branch vessels (e.g., coronary arteries) if the origin is compromised. The incidence of acute aortic dissection has been estimated to range from 2.6 to 3.5 cases per 100,000 person-years, with hypertension identified as one of the primary risk factors [6, 7]. Acute type A dissection (ascending aorta) is twice as common as type B (descending), and surgical management of this more frequently fatal subtype is preferred, whereas medical management is the mainstay of type B dissection not at risk of impending rupture [8].
8.3 Coarctation
Coarctation of the aorta is defined as hemodynamically significant narrowing of the descending aorta, typically at the level of the ductus arteriosus insertion just distal to the origin of the left subclavian artery, distinguishing this entity from pseudocoarctation, which is kinking or buckling of the descending aorta without hemodynamic compromise. Coarctation is estimated to be prevalent in approximately 4 per 10,000 live births, accounting for 4–6 % of all congenital heart defects [9, 10].
8.4 Patent Ductus Arteriosus
Half of term infants undergo ductal closure within the first 24 h after birth, virtually all within 72 h [11]. Delay in closure is inversely proportional to gestational age, and as a result of improvements in neonatal intensive care, the incidence of patent ductus arteriosus has increased [10]. Among term infants, incidence has been estimated between 0.03 and 0.08 % when defined as patency beyond six weeks. Sibling and family studies have demonstrated a genetic predisposition, although no specific genetic marker has been identified [9–12]. The myriad and dynamic effects of this congenital shunt have been well described, and are beyond the scope of this chapter.
8.5 Pulmonary Embolus
This common and often fatal clinical entity is the cause for significant morbidity and mortality (approximately 30 % when untreated) for a large number of patients. Previously, large-scale analyses have estimated the incidence of pulmonary embolus to be 1.5 % from all-cause mortality data, likely a significant underestimation as a large portion of subclinical pulmonary embolus remain undiagnosed [13, 14]. The advent and widespread use of CT angiography has allowed for improved diagnostic sensitivity, and newer data estimate incidence from 62 to 112 cases per 100,000 [14, 15].
8.6 Subclavian Steal
Reversal of flow within a vertebral artery ipsilateral to a hemodynamically significant proximal subclavian artery stenosis is most often an asymptomatic physiologic response to arterial disease. When symptomatic from vertebrobasilar insufficiency or, less commonly, limb ischemia; subclavian steal syndrome is manifest [16]. In a prospective cohort study of patients with asymptomatic neck bruits, 9 % were found with severe subclavian stenosis, and of these patients 64 % were found with subclavian steal syndrome – all with symptoms of vertebrobasilar insufficiency [17]. In a more recent large-scale prospective study [18], 6.5 % of patients undergoing carotid duplex scans were found with significant right/left arm pressure differential, with complete (persistent) steal occurring in 61 % of those patients. Only a small percentage of those with steal were symptomatic, again overwhelmingly from vertebrobasilar insufficiency. 7 of 38 symptomatic patients underwent intervention.
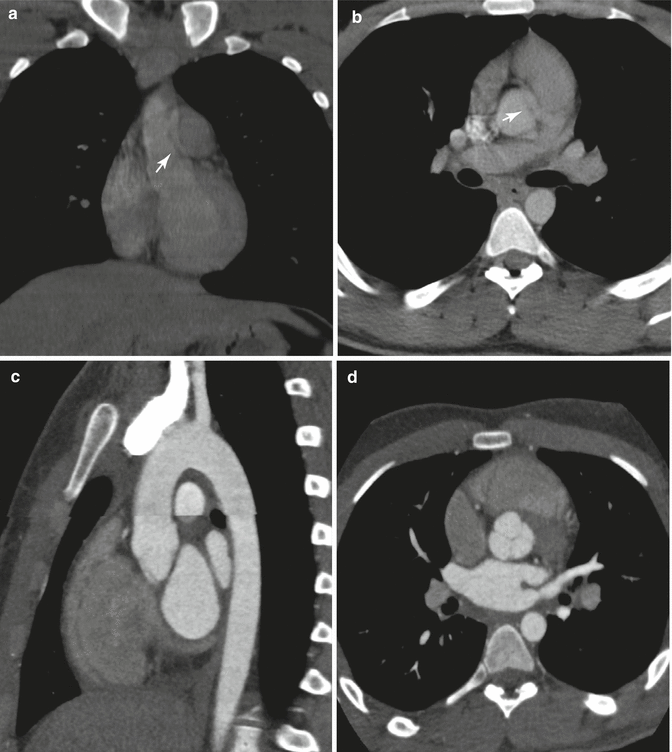
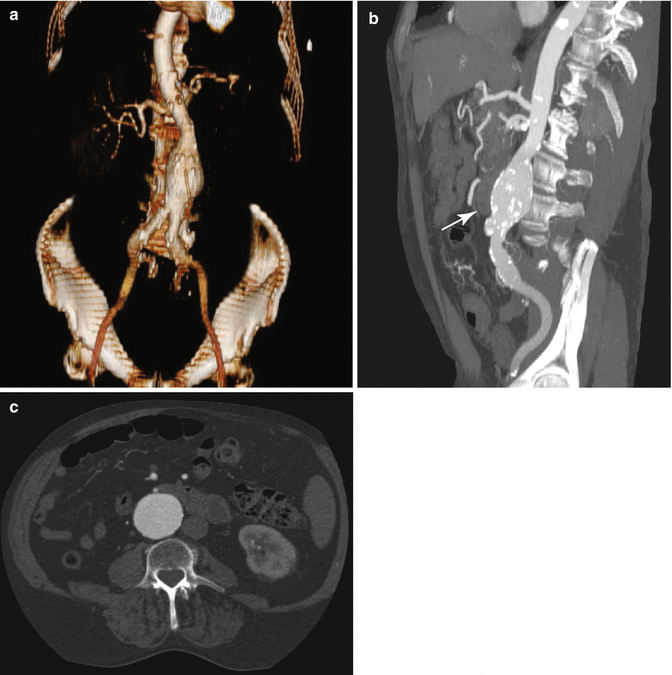
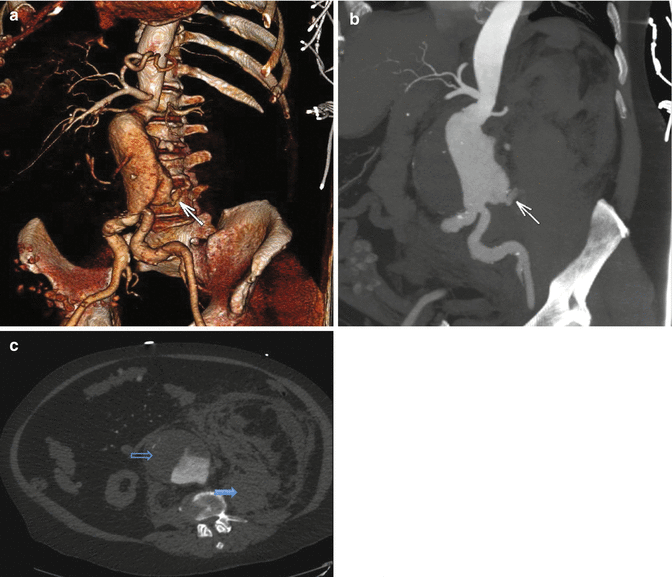
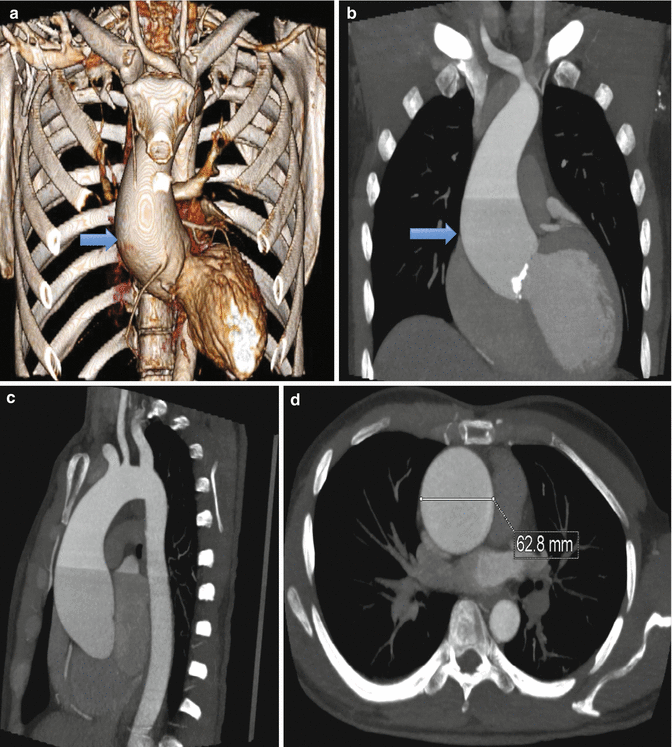
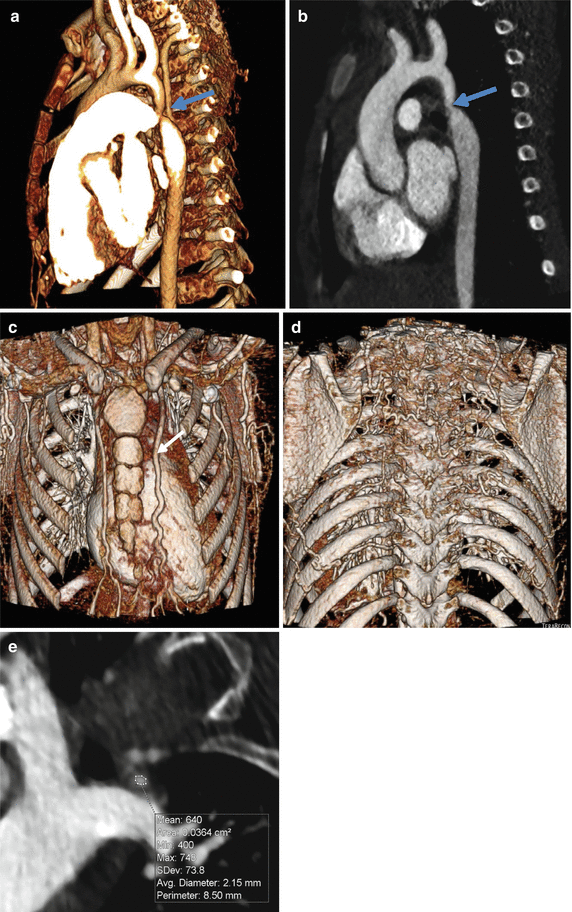





Fig. 8.1
Nongated aortogram in coronal (a) and axial (b) planes, demonstrating an apparent intimal flap (white arrows) at the aortic root, similar in appearance to aortic dissection. This artifact is due to cardiac and aortic motion, and absence of a true dissection can be confirmed with an appropriately timed, gated study. Gated aortogram in sagittal (c) and axial (d) planes in the same patient, obtained to rule out aortic dissection artifactually suggested from the prior figure. Although multi-detector technique allows for exceptionally fast volumetric acquisition, this advantage is not fully realized without gated coordination, capturing images free of motion during the cardiac cycle. Furthermore, multiplanar evaluation is critical to distinguish valve leaflet from luminal flap, which can have similar appearance especially on the axial plane

Fig. 8.2
3D volume rendered (a), sagittal maximum intensity projection (b), and axial (c) reconstructions demonstrating aneurysmal dilatation of the infrarenal aorta. Aortic diameter is normally up to 1.5 cm, with increasing risk of rupture with increasing size and/or rate of growth. Additionally noted is a small luminal outpouching along the anteroinferior aspect, compatible with an ulcerated plaque (white arrow). The presence of calcified plaque along the wall of the ulcer assists in identifying this coincident entity

Fig. 8.3
3D volume rendered (a), sagittal maximum intensity projection (b), and axial (c) reconstructions demonstrating contrast extravasation along the posterior wall of an aneurysmal infrarenal aorta (white arrow), resulting in a large retroperitoneal hematoma (blue arrow). Note the smooth anterior luminal wall is delineated by a large thrombus (black arrow)

Fig. 8.4
3D volume rendered (a), coronal maximum intensity projection (b), sagittal maximum intensity projection (c), and axial (d) reconstructions demonstrating fusiform dilatation of the ascending aorta (blue arrow). Ascending aortic diameter is normally up to 3.5 cm, with increasing risk of rupture with increasing size and/or rate of growth

Fig. 8.5
3D volume rendered (a, c, d) and sagittal (b) reconstructions, with semi-automated vessel analysis (e), demonstrating extensive collateralization and associated enlargement of the left internal mammary artery (white arrow) (c). Post-stenotic dilatation of the decending aorta gives the characteristic “figure 3” appearance on sagittal view (a, b), with focal narrowing centrally (blue arrow). Vessel analysis (e) allows quantification of the degree of stenosis, which differentiates this entity from pseudocoarctation, wherein there is elongation and buckling of the aorta without hemodynamic compromise
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


