Fig. 54.1
Natural history in untreated severe AS
The four key features of the physical exam include palpation of carotid upstroke, auscultation of the systolic murmur, assessment of the splitting of the second heart sound, and examination for signs of heart failure. In severe AS, ventricular systole becomes prolonged and the carotid upstroke is slow rising and late peaking (pulsus parvus et tardus). Rarely, a systolic thrill in carotid pulse may be appreciated. A sustained bifid left ventricular impulse indicates concomitant left ventricular hypertrophy. The classic murmur of AS is a crescendo-decrescendo systolic murmur heard best at the cardiac base (right upper sternal border) radiating to the carotids. The intensity of the murmur does not correlate with the severity of AS. However, as the severity of AS increases, the duration of the murmur may increase, with a peak in mid to late systole. The murmur may rarely be heard in the LV apex area, mimicking mitral regurgitation (Gallavardin’s phenomenon). A diastolic murmur may be heard, if coexistent aortic regurgitation (AR) is present. With severe AS, the physiologic splitting of S2 may be absent as a result of calcification and immobility of aortic valve leading to an inaudible A2. The vigorous atrial contraction may give rise to an S4. Presence of S4 in a young patient with AS is usually consistent with a significant aortic stenosis. Systolic clicks are often heard before the onset of murmur in young patients with bicuspid aortic valves. If heart failure results from severe AS, the systolic murmur may get softer or even be absent due to a low-flow state. Other signs of heart failure such as lateral displacement of the apical impulse, third heart sound, or an elevated jugular venous pressure may be seen on exam [5, 43].
54.2.3.2 Diagnostic Testing
The electrocardiogram (ECG) is typically nondiagnostic in patients with AS. About 85 % of patients with severe AS have LVH with repolarization. Other ECG abnormalities include left atrial abnormality, left or right axis deviation, left bundle branch block, or atrial fibrillation. The chest radiograph is nonspecific in patients with AS. There is rounding of the LV borders and apex consistent with LVH. There may be aortic valve and aortic root calcifications, best appreciated in the lateral projections or fluoroscopy. Dilatation of aorta is sometimes seen.
Echocardiography with Doppler evaluation is the imaging test of choice to diagnose and to estimate the severity of AS. This will help assess the LV dimensions, extent of LVH, LV systolic function, LA dimensions, diastolic function, aortic root dimensions, anatomy of the aortic valve to delineate if the valve is bicuspid or tricuspid, amount of aortic valve calcification, transvalvular pressure gradient, aortic valve area, and associated mitral valve disease. In patients with bicuspid aortic valves, doming of the aortic valve due to asymmetric size of leaflets as well as ascending aortic dilatation is often seen [44]. Table 54.1 delineates the echocardiographic measures used to determine the severity of AS [45].
Table 54.1
Recommendations for classification of aortic stenosis severity by echocardiography
Aortic sclerosis | Mild AS | Moderate AS | Severe AS | |
|---|---|---|---|---|
Aortic jet velocity | ≤2.5 m/s | 2.6–2.9 | 3.0–4.0 | ≥4.0 |
Mean gradient | <20 | 20–40 | >40 | |
AVA (cm2) | >1.5 | 1.0–1.5 | <1.0 | |
Indexed AVA (cm2/m2) | >0.85 | 0.60–0.85 | <0.6 | |
Velocity ratio | 0.50 | 0.25–0.50 | <0.25 |
Exercise stress testing is absolutely contraindicated in symptomatic patients with severe AS. However, there is a role in testing asymptomatic patients with severe AS to provoke symptoms, to ensure that there is an appropriate increase in blood pressure with exercise, and to evaluate exercise capacity [46, 47]. In patients with low cardiac output (ejection fraction, EF <50 %), the calculated gradient and the aortic area may be underestimated due to low aortic flow. In cases of “low-flow, low-gradient” cases of AS, low-dose dobutamine may be necessary to assess the true severity of AS [48, 49]. Diagnosis of low-flow, low-gradient severe AS with a normal ejection fraction is extremely challenging. This is often seen in elderly women with small LV dimensions, LVH, and diastolic dysfunction. In this population, the stroke volume index is <35 ml/m2 with an indexed aortic valve area of <0.6 cm2 [50].
Because of the accuracy of echocardiography in detecting and determining the severity of AS, invasive methods such as cardiac catheterization are seldom necessary in the evaluation of AS. However, it may be helpful in patients in whom the noninvasive tests are inconclusive or if there is a discrepancy in assessing the severity of AS. Coronary angiography is used to determine concomitant coronary heart disease (CHD) prior to surgical aortic valve replacement.
54.2.4 Treatment
Treatment decisions are based on symptoms, severity of valve obstruction, and presence of LV adaptation to pressure overload. The presence of symptoms drives the treatment in patients with AS. Figure 54.2 delineates the treatment algorithm for patients with severe AS [1].
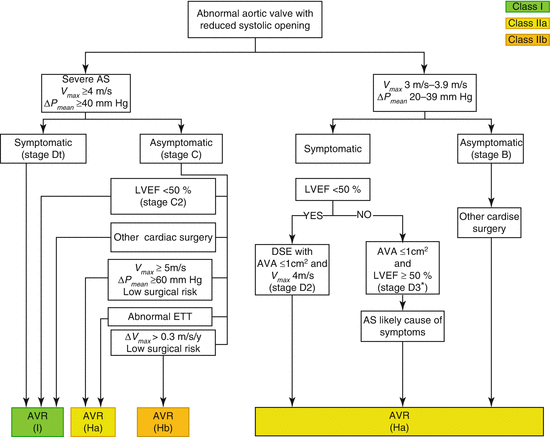

Fig. 54.2
Indications for AVR in patients with AS. Periodic monitoring is indicated for all patients in whom AVR is not yet indicated, including those with asymptomatic AS (stage D or C) and those with low-gradient AS (stage D2 or D3) who do not meet the criteria for intervention. *AVR should be considered with stage D3. AS only if valve obstruction is the most likely cause of symptoms, stroke volume index is <35 ml/m2, indexed AVA is ≤0.6 cm2/m2, and data are recorded when the patient is normotensive (systolic BP <140 mmHg). AS indicates aortic stenosis, AVA aortic valve area, AVR aortic valve replacement by either surgical or transcatheter approach, BP blood pressure, DSE dobutamine stress echocardiography, ETT exercise treadmill test, LVEF left ventricular ejection fraction, ΔPmean mean pressure gradient, and Vmax maximum velocity (Adapted with permission from Ref. [1])
54.2.4.1 Medical Treatment
There are no medical treatments that have shown to delay the progression of AS. Due to the known association between AS and other CHD risk factors, statin therapy has been studied as a potential therapeutic intervention to slow the progression of AS. However, statins have not been shown to delay progression, improve mortality, or delay time to surgery in AS [25, 51, 52]. Diuretics may be useful when symptoms of heart failure have developed, although should be used cautiously as hypovolemia may lower cardiac output and LV end-diastolic pressure and cause orthostatic hypotension. Beta-adrenergic blockers can depress myocardial function and should be avoided in patients with severe AS.
54.2.4.2 Aortic Valve Replacement (AVR)
Calcific AS is now the leading indication for AVR in North America and Europe. There is overwhelming data to indicate that AVR is indicated for patients with symptomatic severe AS [40, 41, 53, 54]. Typical initial symptoms appear to be decreased exercise tolerance and exertional dyspnea. The classic triad of angina, syncope and heart failure are typically symptoms suggestive of advanced AS. Class I indications for AVR are severe symptomatic AS, asymptomatic patients with severe AS and LVEF <50 %, and patients with severe AS undergoing cardiac surgery for other indications [1]. Additionally, AVR could be considered in asymptomatic patients with severe AS when exercise testing provokes symptoms or causes a fall in systolic blood pressure or when markers of rapid progression are present (such as severe valve calcification or increase in aortic velocity of ≥0.3 m/s per year). AVR is also reasonable in asymptomatic patients with very severe AS (Vm ≥5 m/s or mean gradient ≥60 mmHg) who are at a low surgical risk and low-flow low-gradient severe AS with reduced EF [1, 55].
Surgical AVR is considered to be the treatment of choice except in cases of high surgical mortality or inoperable cases. Overall, 30-day surgical mortality is less than 3 % for isolated AVR and approximately 4.5 % for AVR with coronary artery bypass grafting performed at the same time as AVR. Surgical mortality for AVR rises to 10 % in individuals older than the age of 75.
Percutaneous balloon valvulotomy may be considered in children and adolescents with noncalcified aortic valves. Given the high rate of restenosis and failure to improve long-term survival, this is not the procedure of choice in adults who are suitable for AVR. However, it can be occasionally considered for patients who are highly symptomatic as a bridge to surgery in critically ill patients with advanced AS or as palliative procedure when surgery is too high risk.
Transcatheter aortic valve replacement (TAVR) by percutaneous transfemoral or transapical approach is an alternative in selected patient populations who are considered to be at a prohibitive risk for surgical aortic valve replacement. In patients with severe AS, who are poor surgical candidates, and with a predicted post-procedure survival of greater than 12 months, TAVR is a class I recommendation according to the ACC/AHA guidelines. It is also a reasonable alternative to surgical AVR in patients who have high surgical risk and meet an indication for AVR [1, 54]. However, these decisions are made by a Heart Valve Team consisting of an integrated, multidisciplinary, collaborative group of healthcare professionals with expertise in valvular heart disease, cardiac imaging, interventional cardiology, cardiac anesthesia, and cardiac surgery.
Long-term anticoagulation is necessary for mechanical aortic valve prostheses, but not necessary with bioprostheses. Short duration of anticoagulation up to 3 months following a bioprosthetic aortic valve implantation is frequently utilized. Adjunctive pharmacologic therapy with TAVR primarily consists of heparin during the procedure and dual-antiplatelet therapy with aspirin and clopidogrel for 6 months after implantation.
54.3 Aortic Regurgitation (AR) (See Also Chap. 57)
54.3.1 Etiology
The etiology of AR can be divided into two major categories: abnormalities of the aortic leaflets and abnormalities of the supporting structures such as the aortic root and the annulus that prevent proper coaptation of aortic leaflets. In the developing world, rheumatic disease is the most common cause of AR. In the western world, severe AR is most often caused by congenital or degenerative causes. The causes of AR are listed in Table 54.2 [1, 3, 56].
Table 54.2
Etiologies of aortic regurgitation
Aortic leaflet abnormalities | Aortic annulus or root abnormalities |
|---|---|
Congenital abnormalities include bicuspid, unicuspid, or quadricuspid valves Rupture of a congenitally fenestrated valve Subaortic membranes Structural deterioration of bioprosthetic aortic valve | Idiopathic aortic root dilatation, degeneration of the extracellular matrix due to Isolated condition Related to Marfan syndrome Related to congenitally bicuspid aortic valves Ehlers-Danlos syndrome Osteogenesis imperfecta Syphilitic aortitis |
Rheumatic heart disease with fusion of the commissures and retraction of the aortic valve leaflets due to scarring and fibrosis Myxomatous infiltration of the aortic valve; tumors Infective endocarditis Atherosclerotic degeneration Connective tissue disorders such as Marfan syndrome Ingestion of ergot-derived compounds Inflammatory diseases such as aortitis Antiphospholipid syndrome Use of anorectic drugs | Aortitis noted with other connective tissue diseases such as Ankylosing spondylitis Giant cell arteritis Behçet syndrome Psoriatic arthritis |
Systemic disorders that may affect the aortic valve include Lupus erythematosus Giant cell arteritis Takayasu arteritis Ankylosing spondylitis Jaccoud arthropathy Whipple disease Crohn’s disease | Other forms of arthritis associated with Ulcerative colitis Relapsing polychondritis Reiter syndrome |
With leaflet causes such as bicuspid valves, rheumatic fever, or infective endocarditis, there may be a loss of proper coaptation of the cusps, prolapse of the aortic cusp, or incomplete closure of the valve leading to AR (Fig. 54.3). In aortic root enlargement, aortic annulus becomes dilated causing the aortic leaflets to separate and can cause tension and bowing of the cusps, leading to AR.
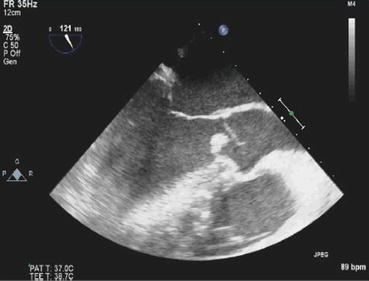

Fig. 54.3
Aortic valve vegetation (group B Streptococcus) causing leaflet prolapse
The overall prevalence of AR is estimated to be between 4.9 % and 10 % [58, 59], with prevalence increasing with age and with more severe AR seen in men [60]. AR due to aortic root disease may be more common than due to valvular disease. In one study that looked at US patients who underwent aortic valve replacement for AR, the cause of AR was valvular in 46 % and aortic root in 54 % of the patients [61].
54.3.2 Pathophysiology and Natural History
AR creates both a volume and pressure overload on the LV. In AR, the LV stroke volume is a combination of forward stroke volume and the regurgitant volume. Although the regurgitant volume empties back into LV during diastole, a large total ejected stroke volume during systole creates a wide pulse pressure and systolic hypertension.
Normal LV forward stroke volume is maintained by increasing the total stroke volume (hence increasing preload) corresponding to the severity of regurgitation. This increase in stroke volume is possible because of increased end-systolic and end-diastolic volumes. Elevated end-diastolic volume increases LV wall stress, thereby increasing LV afterload. In addition, the large total ejected stroke volume that results in systolic hypertension can further increase afterload. In fact, the wall stress in AR is as much as the wall stress noted with AS which is a pure pressure overload condition [62, 63]. This combination of preload and afterload increase eventually results in LV dilation with resultant systolic dysfunction [56, 64]. Volume overload (preload) leads to compensatory eccentric hypertrophy (with replication of sarcomeres in series), while pressure overload (afterload) leads to superimposed compensatory concentric hypertrophy. These mechanisms help to maintain normal wall stress and LV ejection fraction, and patients can remain asymptomatic for a number of years.
With progressive LV dilatation, preload reserves may exhaust or compensatory hypertrophy may be insufficient resulting in afterload mismatch and LV dysfunction. This process can be initially reversible with the correction of the valve disorder but, if left untreated, can lead to irreversible LV dysfunction. In later stages, right-sided pressures can also increase. As heart failure develops, a common initial symptom is shortness of breath, which first develops with exercise and can later occur at rest. Anginal chest pain can also occur due to decreased diastolic perfusion pressure and increased LV oxygen demand due to hypertrophy [56, 64].
Asymptomatic mild, moderate, or even severe AR has a favorable prognosis, with quantitative measurements of AR severity, LV size, and systolic function being predictors of poor outcome. In one study, patients with mild AR had a 10-year survival of 95 ± 4 %, while those with severe AR had a 10-year survival of 69 ± 9 % [65]. Patients with severe chronic AR have an increased risk of morbidity and mortality. Within 10 years of diagnosis of severe AR, heart failure can occur in almost half the patients and most surviving patients require AVR. Once symptoms develop, disease course rapidly worsens. Death may occur within 4 years of onset of angina and within 2 years of onset of heart failure [60].
Unlike chronic AR, acute AR is often a catastrophic illness as the LV has not had the time to develop the compensatory mechanisms described above. Thus, the large total stroke volume and the attendant wide pulse pressure responsible for most of the signs of chronic AR are absent [66]. Compensatory tachycardia attempts to maintain cardiac output [17]. Patients with severe acute AR have a high mortality when treated medically. Rapid diagnosis and prompt surgical intervention are important.
54.3.3 Diagnosis
54.3.3.1 Symptoms and Physical Examination
The presenting symptoms of AR generally are determined by the rapidity with which the regurgitation develops. In chronic AR, exertional dyspnea and fatigue are the most common symptoms. Patients with chronic AR may remain asymptomatic for many years. LV failure is a late event and may be sudden in onset. Patients with acute AR, on the other hand, may present with tachypnea, tachycardia, and dyspnea due to pulmonary congestion/pulmonary edema or may present with cardiogenic shock in severe acute AR.
In patients with chronic AR, findings on physical examination are related to increased stroke volume and widened pulse pressure. The augmented stroke volume can create a hyperdynamic apical impulse and an audible systolic thrill at the cardiac base or carotid arteries. In chronic AR, a patient’s head may bob with each heartbeat (de Musset sign), nail beds may pulsate with minimal pressure (Quincke’s pulse), diastolic bruit may be heard over the femoral artery (Duroziez’s sign), and systolic pulsations of the patient’s uvula may be seen (Muller sign). The peripheral pulses can demonstrate an abrupt rise in upstroke with a quick collapse (water hammer or Corrigan pulse), bisferiens pulse may be palpable, and “pistol shot” sounds may be heard over the femoral artery. The systolic blood pressure may be ≥30 mmHg higher in the leg than in the arm (Hill sign). The heart sounds are usually altered in patients with chronic AR. Although S1 is often normal, S2 is loud or soft depending on the etiology of the AR. A loud closure sound is associated with a dilated aortic root. A soft S2 is found when the AR is due to abnormally thickened and retracted leaflets [57]. Ejection clicks can be heard in young patients with bicuspid valves. The presence of an S3 may signify a failing left ventricle. The classic murmur of AR is a high-pitched, blowing, decrescendo diastolic murmur heard best at the aortic region. In mild AR, murmur is only in early diastole, but as severity of AR worsens, the murmur can become more holodiastolic. In severe AR, a second low-pitched diastolic murmur can be heard at the apex (Austin Flint murmur), which is thought to represent physiologic mitral stenosis (MS) murmur caused by a rapid increase in LV diastolic pressure.
The physical examination findings in acute AR may be quite difficult to elucidate since the ventricle has not had time to compensate. Thus, the large stroke volume and wide pulse pressure, which are responsible for the physical exam findings of chronic AR, are not present. With the sudden decrease in forward stoke volume, the cardiac output can only be maintained by an increase in heart rate. The physical exam is of a quiet precordium, a soft first heart sound, and a short diastolic murmur. The soft S1 is due to tricuspid valve contribution as there is early closure of the mitral valve due to a rapid increase in the LV filling pressure resulting from the large regurgitant volume entering the normal-size LV. Thus, early closure of the mitral valve noted on echo is a poor prognostic sign and should prompt rapid surgical correction.
54.3.3.2 Diagnostic Testing
In patients with chronic AR, signs of left ventricular hypertrophy can be seen on an electrocardiogram. Chronic AR can also lead to left axis deviation, a pattern of LV volume overload with an increase in initial forces, prominent Q waves in leads I, aVL, and V3–V6. With time, these forces diminish with an increase in QRS voltage. A chest radiograph may demonstrate an enlarged cardiac silhouette and enlarged aorta, if present.
Echocardiography is the most useful diagnostic tool in helping to identify both the cause as well as the severity of AR. It allows for the anatomic evaluation of the aortic valve, such as detecting congenital abnormalities (e.g., bicuspid valve), determining if there is a thickening of the valve cusps, or identifying a flail leaflet or vegetation. It is also useful in the measurement of LV end-diastolic and end-systolic dimensions and volumes, LV ejection fraction, and LV mass. Doppler echocardiography is used for the assessment of severity of AR. It is possible to quantify the effective regurgitant orifice and regurgitant volume. The classification of severity of AR using Doppler echocardiography is described in Table 54.3. If the patient has a poor acoustic window, alternative diagnostic methods such as transesophageal echocardiography may be considered.
Table 54.3
Classification or the severity of aortic regurgitation by echocardiography
Variable | Mild | Mild to moderate | Moderate to severe | Severe |
|---|---|---|---|---|
Width of vena contracta (mm) | <3.0 | 3.0–5.9 | 3.0–5.9 | ≥6 |
Ratio of width of aortic regurgitant jet to LV outflow (%) | <25 | 25–44 | 45–64 | ≥65 |
Regurgitant volume (ml per beat) | <30 | 30–44 | 45–59 | ≥60 |
Regurgitant fraction (%) | <30 | 30–39 | 40–49 | ≥50 |
Effective regurgitant orifice (mm2) | <10 | 10–19 | 20–29 | ≥30 |
Cardiac catheterization is mostly used as a preoperative tool to identify preexisting coronary artery disease or to evaluate the coronary and aortic root anatomy. Invasive assessment of LV function and severity of AR is only used in those patients whose noninvasive measurements are inconclusive [1, 56]. Cardiac MRI provides highly accurate measurements of LV volumes, mass, and ejection fraction and allows for a thorough visualization of the aortic root. It also gives information on regurgitant volumes and flow [54, 56, 67, 68].
54.3.4 Treatment
54.3.4.1 Medical
In patients with chronic AR, no medical therapy has been proven to slow progression of disease. Treatment for hypertension is recommended in patients with chronic AR with dihydropyridine calcium channel blockers (Chap. 37) or ACE inhibitors/ARBs (Chap. 36) [1]. Vasodilators such as hydralazine, ACE inhibitors, and calcium channel blockers have been studied given their potential to reduce afterload in patients with chronic AR, theoretically increasing forward flow, decreasing wall stress, and limiting LV dilation and hypertrophy. Long-term studies have not shown consistent improvements in blood pressure, LVEF, LV end-systolic or diastolic dimensions, or other hemodynamic or structural parameters [69]. However, there is a role for medical therapy with vasodilators in symptomatic patients who are not candidates for surgery to help alleviate symptoms. Patients should be referred for surgery when symptoms or LV dysfunction develop.
54.3.4.2 Surgical
Patients with chronic AR can remain asymptomatic for years with normal LV systolic function. Asymptomatic patients with normal LV systolic function have a favorable prognosis. However, once symptoms develop in patients with chronic severe AR, mortality significantly increases without AVR. In a series of 246 patients with severe AR treated only medically, those with NYHA class III–IV had a mortality rate of 24.6 % per year. Even those with class II symptoms had a mortality rate of 6.3 % per year [1, 60] (Fig. 54.4). Symptoms are a major predictor of survival and a class I indication to pursue AVR, regardless of LV function in chronic severe AR. Besides symptoms, resting LV dysfunction (EF <50 %) in the absence of other causes for systolic dysfunction is also a class I indication for surgical AVR. When patients develop asymptomatic LV dysfunction, most will become symptomatic and require AVR within 2–3 years. Outcomes of surgery are better when AVR is performed before the onset of LV systolic dysfunction. Similarly, AVR is a class I indication for patients with severe AR while undergoing cardiac surgery for other indications [1]. Figure 54.5 outlines management algorithm for chronic AR.
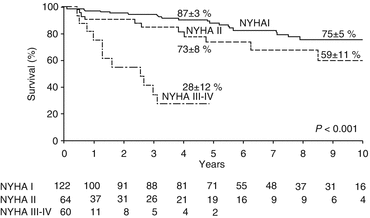
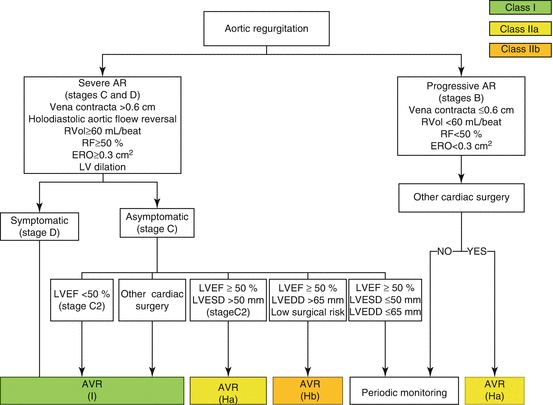

Fig. 54.4
Survival in patients with severe AR stratified according to NYHA class at baseline (Adapted with permission from Ref. [60])

Fig. 54.5
Indications for AVR in chronic AR. AR aortic regurgitation, AVR aortic valve replacement (valve repair may be appropriate in selected patients), ERO effective regurgitant orifice, LV left ventricular, LVEDD left ventricular end-diastolic dimension, LVEF left ventricular ejection fraction, LVESD left ventricular end-systolic dimension, RF regurgitant fraction, RVol regurgitant volume (Adapted with permission from Ref. [1])
54.4 Mitral Stenosis (MS)
54.4.1 Prevalence and Causes
The most common cause of mitral stenosis (MS) worldwide is rheumatic fever, though its prevalence is decreased in industrialized countries [70–72]. Isolated MS is more common in women than in men, with a ratio of 2:1 [72–74]. Other less common causes of MS include congenital anomalies such as parachute mitral valve or double-orifice mitral valve, prior chest radiation exposure, severe mitral annular calcification, mucopolysaccharidoses, and inflammatory diseases such as systemic lupus erythematosus [75], and conditions that mimic mitral stenosis such as left atrial myxoma and cortriatratum.
54.4.2 Pathophysiology and Natural History
Normal mitral leaflets are thin and very mobile. Rheumatic fever is a hypersensitivity reaction induced by group A streptococci in which antibodies directed against the M proteins of certain strains of streptococci cross-react with tissue glycoproteins in the valves. This chronic inflammation results in fibrous thickening and calcification of the valve leaflets, fusion of the commissures, and subvalvular chordal apparatus resulting in chordal thickening, shortening, and fibrosis, all resulting in narrowing of the mitral valve orifice leading to mitral stenosis. The stenotic valve poses obstruction to left atrial emptying, leading to pulmonary hypertension and compromised right ventricular function.
A normal mitral valve (MV) area is 4.0–6.0 cm2. A gradient across the MV is rare unless the valve is less than 2 cm2. Exertional symptoms usually develop when the valve area decreases below 2.0–2.5 cm2. Transmitral gradients that result in symptoms are typically based on the MV area, cardiac output, and duration of diastole. Atrial contraction helps maintain flow across the stenotic MV. Atrial fibrillation therefore has an adverse effect and precipitate dyspnea and heart failure.
Pulmonary hypertension (PH) is the result of backward pressure that is transmitted from the left atrium and pulmonary arteriolar vasoconstriction due to intimal hyperplasia and medial hypertrophy. In some patients, a secondary obstruction may also develop at the level of the pulmonary veins. Once these changes occur, despite correction of MS, at least a part of PH becomes irreversible (Chap. 45).
Resting symptoms can develop when the valve area is less than 1.5 cm2. However, symptoms occur in patients with large valve areas if conditions arise that decrease diastolic filling or increase transmitral flow. Such conditions commonly include atrial fibrillation, pregnancy, exercise, infection, or emotional stress. Once significant symptoms develop, however, survival decreases to 0–15 % at 10 years [76]. If marked pulmonary hypertension develops, the average survival is less than 3 years [77]. The severity of MS is defined in Table 54.4 [1, 45].
Table 54.4
Grading mitral stenosis severity
Mild | Moderate | Severe | |
|---|---|---|---|
Mean gradient (mm Hg) | <5 | 5–10 | >10 |
Pulmonary artery systolic pressure (mm Hg) | <30 | 30–50 | >50 |
Valve area (sq.cm.) | >1.5 | 1.0–1.5 | <1 |
54.4.3 Diagnosis
54.4.3.1 Symptoms and Physical Examination
Patients with MS often are asymptomatic until the onset of atrial fibrillation or during pregnancy when they experience dyspnea and orthopnea. Symptoms of MS are usually related to the elevated left atrial pressure and reduced cardiac output due to a fixed obstruction to the filling of left ventricle. The first symptoms of MS are usually exertional dyspnea and fatigue. However, patients may also present with hemoptysis, chest pain due to pulmonary hypertension, pulmonary edema, endocarditis, atrial fibrillation, or an embolic event. Rarely patients may present with hoarseness due to compression of recurrent laryngeal nerve from left atrial enlargement (Ortner’s syndrome), hemoptysis, or dysphagia. Survival is good (80 % in 10 years) in patients who are symptomatic or minimally symptomatic.
The classic physical examination in patients with MS includes a normal apical LV impulse as left ventricle is not affected in isolated or pure MS. However, a right ventricular heave may be present due to right ventricular hypertrophy. Auscultation reveals an accentuated first heart sound (S1) in patients with a mobile but thickened valve, an opening snap (OS) followed by a mid-diastolic murmur, and a presystolic murmur heard best at the apex in the left lateral decubitus position. These findings, however, may not be present in patients with severe pulmonary hypertension, with low cardiac output, or with a heavily calcified and immobile valve. The mid-diastolic, low-frequency murmur of MS is heard best with the bell of the stethoscope with the patient in the left lateral decubitus position. The second sound can also be loud because of an increased P2 component thought to result from pulmonary hypertension, with resultant right ventricular overload. In severe MS with low flow across the mitral valve, the murmur may be soft and difficult to hear, especially in patients with atrial fibrillation. “Mitral facies” may manifest as purple-pink patches on the cheeks as a result of systemic vasoconstriction from the low cardiac output state of mitral stenosis.
54.4.3.2 Diagnostic Testing
The electrocardiogram can be completely normal especially in patients with mild MS. Patients in sinus rhythm show evidence of left atrial enlargement. Atrial fibrillation is also a common finding. ECG evidence of right axis deviation, right ventricular hypertrophy, and right atrial enlargement can be seen in individuals with pulmonary hypertension. The most common chest x-ray finding in patients with significant MS is that of left atrial enlargement. Enlargement of the right atrium, right ventricle, and the pulmonary artery can also be seen on chest x-rays of patients with advanced MS with pulmonary hypertension.
Echocardiogram is the primary imaging tool used to assess a patient with MS. The anterior leaflet generally shows a “hockey-stick” or “bent-knee” deformity since restricted motion of the valve most commonly involves the leaflet tips. The posterior leaflet is often restricted in both systole and diastole. The echo also provides information about the size of the left atrium and the size and function of the left ventricle and the right-sided chambers (Figs. 54.6 and 54.7). Doppler examination provides information about the severity of MS by assessment of transvalvular pressure gradient, mitral valve area, the presence of other associated valve lesions, and the degree of pulmonary hypertension [78–82]. Echocardiography is also helpful to diagnose atrial myxomas that can have physical findings similar to those with MS. Transesophageal echocardiogram is more sensitive than transthoracic echocardiogram to assess left atrial appendage thrombi. Wilkins scoring system is a simple echocardiographic scoring system that takes into account the amount of leaflet thickening, the degree of leaflet mobility, the involvement of subvalvular apparatus, and the degree of calcification to decide whether a valve is suitable for percutaneous balloon valvuloplasty [83]. A Wilkins score less than 8 predicts a good result following an intervention and improved survival [84].
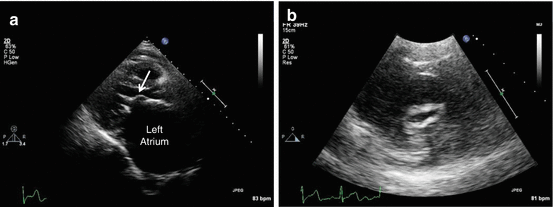
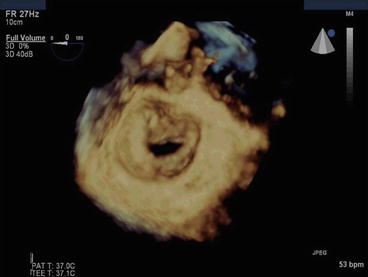

Fig. 54.6
Rheumatic mitral stenosis. Note the thickening of mitral leaflets with “hockey-stick” appearance of anterior mitral leaflet (arrow) and an enlarged left atrium (L). The short-axis image of mitral valve demonstrates a “fishmouth” appearance with valve area of 0.9 cm2 (R)

Fig. 54.7
3D Echo image of rheumatic MS with a valve area of 0.8 cm2
Routine diagnostic cardiac catheterization is no longer performed in patients with MS since accurate hemodynamic information is usually obtainable using echo. Diagnostic cardiac catheterization is only necessary when echo is nondiagnostic or results are discrepant with clinical findings. Catheterization-based hemodynamic assessment is also performed before, during, and after percutaneous balloon valvotomy [85]. Coronary angiography is performed in patients scheduled to undergo valve replacement surgery if there is a risk of coronary artery disease.
54.4.4 Treatment
Patients with MS caused by rheumatic heart disease should receive penicillin prophylaxis for beta-hemolytic streptococcal infections to prevent recurrent rheumatic fever [86]. Prophylaxis for infective endocarditis is no longer recommended unless the patient has a prior history of endocarditis or a prosthetic valve. Anticoagulant therapy is indicated for prevention of systemic embolism in patients with atrial fibrillation (persistent or paroxysmal), any prior embolic events (even if in sinus rhythm), and in those with documented left atrial thrombus. When mild symptoms appear, diuretics have been shown to be effective in lowering left atrial pressure and reducing symptoms.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


