Fig. 9.1
2-D Echocardiogram parasternal long axis view of the left ventricle revealing prolapse of both the anterior and posterior mitral leaflets (white arrow)
Table 9.1
Classification of the severity of valve disease in adults
A. Left–sided valve disease | |||
Indicator | Aortic stenosis | ||
Mild | Moderate | Severe | |
Jet velocity (m/s) | Less than 3.0 | 3.0–4.0 | Greater than 4.0 |
Mean gradient (mmHg)a | Less than 25 | 25–40 | Greater than 40 |
Valve area (cm2) | Greater than 1.5 | 10–1.5 | Less than 1.0 |
Valve area index (cm2/m2) | Less than 0.6 | ||
Mitral stenosis | |||
Mild | Moderate | Severe | |
Mean gradient (mmHg)a | Less than 5 | 5–10 | Greater than 10 |
Pulmonary artery systolic pressure (mmHg) | Less than 30 | 30–50 | Greater than 50 |
Valve area (cm2) | Greater than 1.5 | 1.0–1.5 | Less than 1.0 |
Aortic regurgitation | |||
Mild | Moderate | Severe | |
Qualitative | |||
Angiographic grade | 1+ | 2+ | 3–4+ |
Color Doppler jet width | Central jet, width less than 25 % of LVOT | Greater than mild but no signs of severe AR | Central jet. width greater than 65 % LVOT |
Doppler vena contracts width (cm) | Less than 0,3 | 0.3–0.6 | Greater than 0.6 |
Quantitative (cath or echo) | |||
Regurgitated volume (ml per beat) | Less than 30 | 30–59 | Greater than or equal to 60 |
Regurgitated fraction (%) | Less than 30 | 30–49 | Greater than or equal to 50 |
Regurgitated orifice area (cm2) | Less than 0.10 | 0.10–0.29 | Greater than or equal to 0.30 |
Additional essential criteria | |||
Left ventricular size | Increased | ||
Mitral regulation | |||
Mild | Moderate | Severe | |
Qualitative | |||
Angiographic grade | 1+ | 2+ | 3–4+ |
Color Doppler jet area | Small. central jet (less than 4 cm2 or less than 20 % LA area) | Signs of MR greater than mild present but no criteria for severe MR | Vena contracts width greater than 0.7 cm with large central MR jet (area greater than 40 % of LA area) of with a wall-impinging jet of any size, swirling in LA |
Doppler vena contracts width (cm) | Less than 0.3 | 0.3–0.69 | Greater than or equal to 0.70 |
Quantitative (cath or echo) | |||
Regurgitant volume (ml/beat) | Less than 30 | 30–59 | Greater than or equal to 60 |
Regurgitant fraction (%) | Less than 30 | 30–49 | Greater than or equal to 50 |
Regurgitant orifice area (cm2) | Less than 0.20 | 0.20–0.39 | Greater than or equal to 0.40 |
Additional essential criteria | |||
Left atrial size | Enlarged | ||
Left ventricular size | Enlarged | ||
B. Right–sided valve disease | Characteristic | ||
Severe tricuspid stenosis: | Valve area less than 1.0 cm2 | ||
Severe tricuspid regurgitation: | Vena contracts width greater than 0.7 cm and systolic flow reversal in hepatic veins | ||
Severe pulmonic stenosis: | Jet velocity greater than 4 m/s or maximum gradient greater than 60 mmHg | ||
severe pulmonic regurgitation: | Color jet fills outflow tract; dense continuous wave Doppler signal with a steep deceleration slope | ||
Trans-esophageal Doppler echocardiography (TEE) is helpful in patients with poor imaging windows. In addition to better delineation of the anatomy of the mitral apparatus, left atrial appendage size and function and pulmonary vein flow can be measured. Flow-reversal in the pulmonary veins suggests severe MR. Exclusion of a left atrial thrombus is important for those with embolic symptoms or who are to undergo cardioversion for an atrial tachyarrhythmia. TEE is essential for the preoperative assessment of patients being considered for mitral valve repair. A complete assessment of the three segments of both leaflets is required. Also the chordae tendineae must be imaged and the dimensions the annulus quantified. While most laboratories still employ two dimensional imaging, the use of three dimensional imaging provides superior spatial assessment and can be very useful to define the optimum surgical approach. Other imaging modalities, i.e. CT scanning and MRI may be useful in particular circumstances [14].
Electrocardiography
The electrocardiogram (ECG) is usually normal in the majority of patients, but a small minority of patients demonstrates nonspecific ST-T wave abnormalities. More severe MR is suggested by the presence of electrocardiographic criteria for both left atrial and ventricular enlargement. Paroxysmal supraventricular tachycardia is the most common tachyarrhythmia encountered in patients with MVP syndrome. However, some patients develop ventricular premature contractions and ventricular tachyarrhythmias due to stretch-induced depolarizations of resident cardiomyocytes and QT-segment prolongation. Finally, left atrio-ventricular bypass tracts, including the Wolf-Parkinson-White syndrome, are associated with MVP [4].
Chest Radiography
Chest radiography is often normal in mild MR, but can demonstrate a constellation of findings as the heart responds to worsening disease. Left ventricular and atrial enlargement can occur, the latter manifest by straightening of the left heart border and splaying and posterior deviation of the left main bronchus. With hemodynamic decompensation, pulmonary venous congestion progressing to pulmonary edema, pulmonary artery enlargement, and right atrial and ventricular enlargement may be seen.
Cardiac Catheterization
Invasive hemodynamic assessment of mitral regurgitation can be further evaluated by a left and right heart cardiac catheterization, especially if the non-invasive assessment is ambiguous or for pre-operative evaluation. Measurement of intracardiac pressures and evaluation of pressure waveforms is essential [2]. Increasing MR severity may be manifest by an increase in average left atrial/pulmonary capillary wedge (PCW) pressures accompanied by a late systolic peak (“v” wave) whose height correlates with MR severity [2]. In addition, a right heart catheterization to exclude pulmonary hypertension and tricuspid valve disease, cardiac output measurements, and calculation of pulmonary total and vascular and systemic vascular resistances. A difference between the PCW and LV diastolic pressures should excluded by simultaneous recording of the pressure waveforms. Left ventriculography is used to quantify the degree of mitral regurgitation. Finally, coronary angiography is required in those with suspected or known coronary artery disease, especially if an ischemic etiology to their MR is suspected, those with multiple CAD risk factors, an underlying cardiomyopathy, and those over 40 years of age.
Clinical Course
The majority of patients with mild MR of any etiology remain asymptomatic. However, MVP patients may report atypical chest pain, syncope, presyncope, and palpitations. Worsening MR is characterized by a relatively long asymptomatic period. During this time mitral apparatus, LV and LA remodeling may occur. LV dilatation and remodeling accompanies worsening MR and can in itself increase the degree of valve dysfunction. The increased wall stress can induce apoptosis and fibrosis leading to a decline in systolic performance associated with pulmonary congestion, dyspnea and other manifestations of CHF. Left atrial enlargement also occurs during this asymptomatic period. The first clinical manifestation may be atrial fibrillation or a systemic embolus. Disease progression can result in the development of pulmonary hypertension due to pulmonary venous hypertension or an elevated pulmonary vascular resistance. Development of systemic congestion due to right sided heart failure and tricuspid regurgitation can manifest as, congestive hepatomegaly, edema and ascites.
Progressive mitral regurgitation results in the development of CHF occur in about 15 % of patients with MVP over a course of 10–15 years [10, 14]. Patients are also at increased risk of developing mitral valve infective endocarditis which can accelerate the progression MR, LA and LV remodeling, decline in LVEF. Those factors but especially a decrease in LVEF to below 60% are associated with reduced symptom free survival [2].
Mitral Stenosis
Rheumatic heart disease has a low prevalence in the United States, primarily due to effective prophylaxis against rheumatic fever. However, it remains an important cause of valve disease in developing countries, and because of this reservoir, rheumatic valve disease is often found in immigrant communities in the United States. Acute rheumatic fever results from an autoimmune-mediated inflammatory response to an inadequately treated infection with a Group A β-hemolytic Streptococcus. A pancarditis results, involving the mural endocardium, myocardium and epicardium, whose major sequellae involves an inflammatory valvulitis and endocarditis involving the mitral, aortic, and tricuspid valves in decreasing relative frequency [2]. Rheumatic heart disease may be viewed as a disease of the mitral valve as there is always involvement of the mitral valve apparatus. In addition, rheumatic mitral disease is two times more common in women than men [2, 5]. Aschoff bodies, pathognomonic for rheumatic disease, are always found when there is involvement of the mitral valve [2]. Acute rheumatic valve disease is characterized by small vegetations and acute valvulitis and is characterized by mitral regurgitation. Mitral stenosis, with or without mitral regurgitation, is a late finding and is characterized by commissural fusion associated with varying degrees of leaflet thickening due to deposition of fibrous tissue and calcium. Calcium deposition in the mitral leaflets and involvement of the aortic valve are more pronounced in men than women, older patients and those with higher arterial blood pressure.
Other causes of mitral stenosis include congenital leaflet and apparatus deformities, e.g. parachute mitral valve, narrowing of the mitral orifice due to exuberant calcification of the mitral annulus, obstruction due to large vegetations or a left atrial myxoma or rarely a cor triatriatum.
Physical Examination
Patients with mitral stenosis may have a characteristic reddening of the cheeks (malar flush). The cardiovascular finding depend on the severity of the stenosis and whether CHF, pulmonary hypertension and/or primary or secondary tricuspid valve disease is present. The left ventricular apical impulse is usually normal to reduced and may be displaced laterally if RV enlargement is present. The first heart sound is often increased in intensity. An increased pulmonic component of S2 can indicate pulmonary hypertension, as can a RV heave or pulmonary diastolic murmur. An apical holosystolic mumur suggests the presence of MR. In patients with a pliable valve, an opening snap (OS) can be heard 60–120 ms after S2. The shorter the S2-OS interval is, the higher the left atrial pressure. An apical diastolic rumble following OS and with pre-systolic accentuation is characteristic of MS. It is best heard with the patient in the left lateral decubitus position. The presence of a left or right parasternal systolic or diastolic murmur that increases with inspiration suggests tricuspid regurgitation or stenosis, respective. CHF is manifested by jugular venous distention. An absent “a” wave suggests atrial fibrillation while a “c-v” wave is consistent with tricuspid regurgitation. Systemic venous hypertension is often manifest by hepatomegaly, ascites, and edema. Wheezing may be an early finding of pulmonary venous hypertension, while rales are often present in pulmonary edema.
Diagnostic Evaluation
Doppler-Echocardiography
Transthoracic Doppler-echocardiography (TTE) is the primary imaging modality to diagnose MS, quantify its severity and assess the mitral valve and apparatus morphology, determine its effect on the other cardiac valves, the morphology and performance of the cardiac chambers, determine prognosis, and plan for surgical or catheter-based intervention. Commissural fusion is characteristic of rheumatic mitral valve disease. The leaflets, especially the anterior one may remain pliable resulting in the characteristic diastolic hockey stick appearance in the 2-D parasternal long axis view. As the disease progresses fibrosis and calcification results in decreased mobility Thickening and calcification extends proximally, in contrast to degenerative disease where the process extend from the annulus to the tips. The orifice is reduced and has a characteristic fish mouth appearance in the parasternal short axis view [2]. Thickening and retraction of the chordae tendineae also affect the mobility and the overall degree of stenosis.
Stenosis severity is quantified by determination of the mean gradient (ΔP) across the valve and apparatus calculated by the modified Bernoulli equation (ΔP = 4v2) and the orifice area (Table 9.1) [15]. The latter can be determined by planimetry performed at the tips of the leaflets in the parasternal short axis view imaged by both 2-D and 3-D echocardiography [2]. Other techniques for quantification of the effective orifice area include the pressure half time PHT (MVA = 220/PHT) and the continuity equation. It is important to recognize that in patients with aortic regurgitation, atrial fibrillation, abnormal left atrial compliance due to left atrial dilatation, abnormal left ventricular compliance, and immediately after balloon valvuloplasty, mitral valve area assessment by PHT has inherent limitations [2]. As mitral regurgitation frequently accompanies stenosis, determination of its severity is an essential part of the Doppler-echocardiographic examination. In addition to assessment of the valve itself, the rest of the mitral apparatus, the atria and ventricles, the other valves, especially the aortic and tricuspid valves, need evaluation.
Trans-esophageal echocardiography (TEE) is employed when TTE imaging is inadequate for diagnosis, to better visualize the mitral apparatus, to diagnose infectious complications, determine the if an intracardiac source of systemic embolus is present e.g. a left atrium or left atrial appendage thrombus. It is also used prior to cardioversion for atrial arrhythmias and as part of the pre-operative and intra-operative assessment of mitral repair surgery or catheter-based intervention. In particular, the Wilkins score derived from a TEE examination of the mitral valve apparatus can be used to predict suitability for mitral balloon valvuloplasty (Table 9.2) [8, 16].
Table 9.2
Determinants of the echocardiographic mitral valve score
Grade | Mobility | Subvalvular thickening | Thickening | Calcification |
|---|---|---|---|---|
1 | Highly mobile valve with only leaflet tips restricted | Minimal thickening just below the mitral leaflets | Leaflets near normal in thickness (4–5 mm) | A single area of increased echo brightness |
2 | Leaflet mid and base portions have normal mobility | Thickening of chordal structures extending up to one third of the chordal length | Midleaflets normal, considerable thickening of margins (5–8 mm) | Scattered areas of brightness confined to leaflet margins |
3 | Valve continues to move forward in diastole, mainly from the base | Thickening extending to the distal third of the chords | Thickening extending through the entire leaflet (5–8 mm) | Brightness extending into the midportion of the leaflets |
4 | No or minimal forward movement of the leaflets in diastole | Extensive thickening and shortening of all chorda structures extending down to the papillary muscles | Considerable thickening of all leaflet tissue (greater than 8–10 mm) | Extensive brightness throughout much of the leaflet tissue |
Cardiac magnetic resonance imaging (MRI) is an additional imaging modality which demonstrates thickening of the leaflets and reduced opening of the mitral valve in diastole in patients with mitral stenosis; and the maximal extent of leaflet opening correlates well with the severity of stenosis [2].
Electrocardiography
The electrocardiogram in patients with MS, although often nonspecific in patients with mild disease, shows characteristic changes in patients with moderate to severe stenosis. The most common findings include left atrial enlargement, atrial fibrillation often with coarse fibrillatory waves. Criteria for right ventricular hypertrophy, e.g. right axis deviation, increased R-wave voltage in the V1-3 and associated ST-T wave abnormalities, may be observed in patients with pulmonary hypertension. The presence of left ventricular hypertrophy/enlargement suggests associated MR or aortic valve disease.
Chest Radiography
Early in the course of disease, the cardiac silhouette may be relatively normal, however, straightening of the left heart border due to left atrial appendage enlargement and splaying and posterior displacement of the left main bronchus may be observed. Mitral valve calcification can sometimes be observed on a plain chest X-ray, and is an indicator of advanced disease and occurs most often in older people. Calcification of the mitral annulus may be visible in older patients with either rheumatic or non-rheumatic degenerative valve disease. Elevation of the pulmonary venous pressure can be manifest by prominence of the upper lobe pulmonary veins progressing to interstitial and alveolar infiltrates. Pulmonary hypertension is manifest by enlargement of the main pulmonary artery and right ventricle, while right atrial enlargement suggests the presence of tricuspid regurgitation and right heart failure. LV enlargement suggests associated mitral regurgitation.
Cardiac Catheterization
Cardiac catheterization is required in mitral stenosis patients in order to accurately measure hemodynamics, to assess the presence of concomitant aortic and tricuspid valve and aortic disease, to quantify associated MR and LV size and systolic performance, and to exclude co-existent coronary artery disease. The latter is especially important in patients with ischemic symptoms, pre-existent disease, multiple CAD risk factors, and those generally ≥40 years of age.
Characteristic hemodynamic abnormalities include an elevated left atrial pressure or pulmonary capillary wedge pressure (PCWP) with a diastolic pressure difference between the left atrium and ventricle. A prominent “a”-wave is often observed in patients in sinus rhythm. Cardiac output should be measured simultaneously with the recording of the pressure difference. The Gorlin formula is used to calculate the mitral valve effective orifice area (EOA) [17]. Both the gradient across the mitral valve orifice as well as the mitral valve orifice area are calculated at the time of catheterization. A mean gradient >10 mmHg and a mitral valve area of <1.0 cm2 defines severe MS, while moderate MS is defined as a gradient of 5–10 mmHg or an EOA of 1.0–1.5 cm2 and mild stenosis patients generally have a gradient of <5 mmHg and EOA >1.5 cm2 (Table 9.1) [8, 10].
It is important to recognize some common pitfalls during invasive hemodynamic assessment of mitral valve stenosis. It is important to record an accurate PCWP tracing when it is used as a surrogate for left atrial pressure. The diastolic filling time affects the transvalvular gradient. Irregularity in the diastolic filling time occurs in atrial fibrillation; ten consecutives beats should be averaged to avoid errors when using the Gorlin formula [17]. Finally it is important to recognize coexistent mitral regurgitation as it will lead to overestimation of the true severity of mitral valve stenosis.
Right ventricular, pulmonary artery pressures, and total pulmonary artery and pulmonary vascular resistance may be normal in mild mitral stenosis, but pulmonary arterial pressures and resistances increase with progression of the disease.
There is often a discrepancy between hemodynamic measurements and reported symptoms. Noninvasive exercise stress can help resolve this problem. A significant increase in the gradient across the mitral valve (>15 mmHg) associated with an increase in the pulmonary artery systolic pressure to >60 mmHg during exercise supports the presence of significant disease and are both indications to consider mitral balloon valvuloplasty [8, 10]. In patients with severe mitral stenosis, pulmonary artery pressures are elevated even at rest. However, this is often a reversible process with eventual decrease in the pulmonary artery pressures after valve replacement or balloon valvotomy.
Clinical Course
Patients with rheumatic mitral valve disease may be asymptomatic for many years. However, progressive dyspnea on exertion resulting in reduced exercise capacity is the usual presenting report. A careful history must be obtained with respect to performance of activities of daily living to uncover symptomatic progression. Frequent episodes of bronchitis or pneumonia may suggest mitral valve disease before symptoms and signs of overt CHF occur. Acute pulmonary edema may be the first manifestation of mitral stenosis. It may be precipitated by the onset of an atrial tachyarrhythmia, often atrial fibrillation, acute infection, or the hemodynamic stress of pregnancy and delivery [18]. Acute pulmonary edema occurring during labor and delivery can be the first clinical manifestation mitral stenosis. Rarely, mitral stenosis may present with a systemic embolus resulting in a CVA or TIA. Acute hemoptysis can be observed in pneumonia, a pulmonary embolus or due to rupture of bronchial-pulmonary artery collaterals.
Treatment of Mitral Valve Disease
Valvular heart disease management is similar in women and men, however specific gender related differences related to disease prevalence, body size, age at presentation, differences in left ventricular response to overload states need additional consideration in the management of women with valvular heart disease [18].
Medical treatment of patient with moderate-severe mitral regurgitation relies on improving the hemodynamic milieu to reduce the severity of MR. Patients with left ventricular enlargement and coaptation failure often respond to reduction in pre- and after-load by the use of vasodilators, e.g. ACE-Is or ARBs, and judicious use of diuretics. Systolic blood pressure should be reduced to therapeutically recommended levels. Patients with severely reduced LV systolic performance should be treated as a dilated cardiomyopathy. MVP patient with preserved LV systolic performance may benefit from beta-blocker therapy. Patients with atrial fibrillation, who are not candidates for cardioversion or pulmonary vein isolation, require rate control and anticoagulation with warfarin. Currently, prothrombin and Factor Xa inhibitors are not approved for use in valve disease patients. Mitral stenosis patients should be anticoagulated with warfarin even if they are in sinus rhythm, as they often experience short burst of atrial fibrillation.
The current guidelines for operative intervention for severe MR are presented in Fig. 9.2. Surgical intervention should be considered in patients with severe mitral regurgitation who become symptomatic with functional decline and an enlarging LV associated with a drop in LVEF. However, because even a modest decline in LVEF below 60 % results in increased long-term morbidity and mortality early surgery is now recommended even before significant symptoms have developed [19]. Also, operative repair should be considered in those with progressive atrial enlargement before atrial fibrillation develops. Mitral valve repair is the procedure of choice for suitable candidates when the operation can be performed by an experienced expert surgical team. The high rate of procedural success combined with very low perioperative complications related to primary mitral valve repair has encouraged earlier operation. A bioprosthetic valve is recommended for those patients who are not candidates for valve repair and are not at high risk for prosthetic valve structural deterioration. Mechanical prostheses are generally reserved for patient at high risk for early bioprosthetic valve structural deterioration and those who would require anticoagulation for atrial fibrillation or other reasons. Patients with ischemic MR should undergo coronary revascularization if myocardial viability is demonstrated.
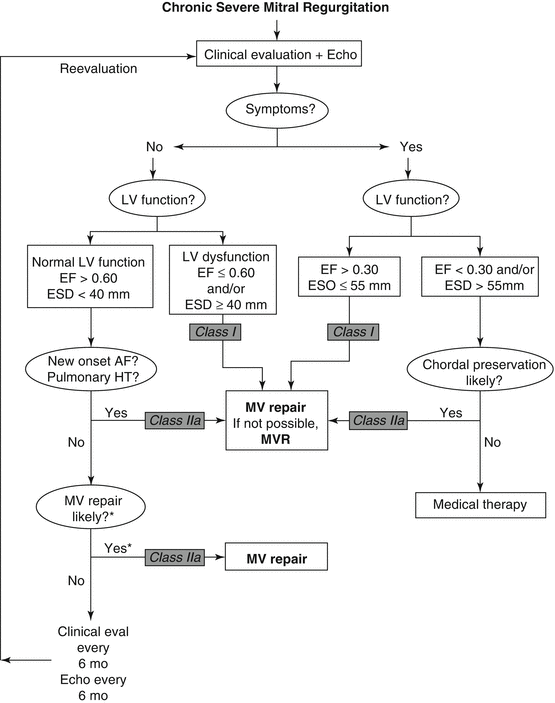

Fig. 9.2
Management strategy for patients with chronic severe mitral regurgitation. *Mitral valve (MV) repair may be performed in asymptomatic patients with normal left ventricular (LV) function if performed by an experienced surgical team and if the likelihood of successful MV repair is greater than 90 %. AF atrial fibrillation, echo echocardiography, EF ejection fraction, ESD end-systolic dimension, eval evaluation, HT hypertension, and MVR mitral valve replacement (Reprinted with permission from Bonow et al. [8])
In patients with organic mitral regurgitation mitral valve replacement is usually the procedure of choice. In contrast, there is significant controversy in regards to the procedure of choice in patients with mitral regurgitation secondary to ischemia. In a recent published study there was no significant difference in operative or overall mortality between mitral valve repair as compared to valve replacement in patients with ischemic mitral regurgitation [20]. In patients with coexistent coronary artery disease, mitral valve repair has been reported by a separate group to be superior to valve replacement with significantly decreased peri-operative morbidity and in-hospital mortality [21].
Percutaneous mitral valve repair with placement of a mitral clip device to approximate the edges of the mitral valve at the site of the regurgitant jet has been recently reported to be safe and efficacious in a randomized trial [22]. Percutaneous repair was however found to be less effective at reducing the degree of mitral regurgitation compared to conventional mitral valve repair or replacement.
Patients with rheumatic mitral stenosis can be treated with percutaneous balloon valvuloplasty, surgical mitral commisurotomy or mitral valve replacement based on the anatomy and functional status of the valve. Women with symptomatic mitral stenosis of at least moderate severity or new onset atrial fibrillation, as well as those patients which are asymptomatic with moderate mitral stenosis and associated pulmonary hypertension or increased transmitral valvular gradient during exercise and suitable valve anatomy, are recommended to undergo percutaneous mitral balloon valvuloplasty mitral valvulopastly is recommended (Fig. 9.3a–c) [5, 8, 10].
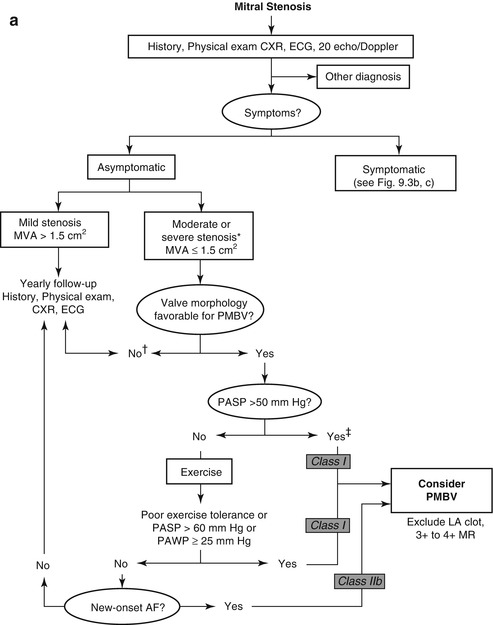
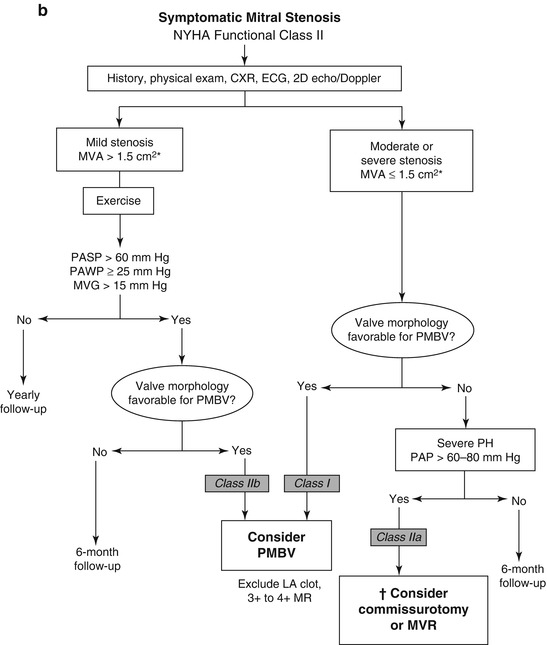
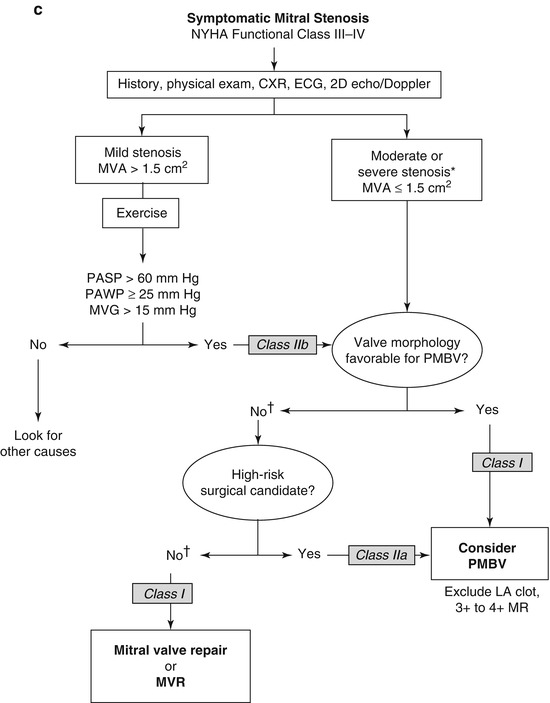



Fig. 9.3
(a): Management strategy for patients with mitral stenosis. †There is controversy as to whether patients with severe mitral stenosis (MVA less than 1.0 cm2) and severe pulmonary hypertension (pulmonary artery pressure greater than 60 mmHg) should undergo percutaneous mitral balloon valvotomy (PMBV) or mitral valve replacement to prevent right ventricular failure. ‡Assuming no other cause for pulmonary hypertension is present. AF atrial fibrillation, CXR chest X-ray, ECG electrocardiogram, echo echocardiography, LA left atrial, MR mitral regurgitation, and 2D 2-dimensional8. (b): Management strategy for patients with mitral stenosis and mild symptoms. *The committee recognizes that there may be variability in the measurement of mitral valve area (MVA) and that the mean transmitral gradient, pulmonary artery wedge pressure (PAWP), and pulmonary artery systolic pressure (PP) should also be taken into consideration. †There is controversy as to whether patients with severe mitral stenosis (MVA less than 1.0 cm2) and severe pulmonary hypertension (PH PP greater than 60–80 mmHg) should undergo percutaneous mitral balloon valvotomy (PMBV) or mitral valve replacement to prevent right ventricular failure. CXR chest X-ray, ECG electrocardiogram, echo echocardiography, LA left atrial, MR mitral regurgitation, MVG mean mitral valve pressure gradient, NYHA New York Heart Association, PAP pulmonary artery pressure, and 2D 2-dimensional8. (c): Management strategy for patients with mitral stenosis and moderate to severe symptoms *The writing committee recognizes that there may be variability in the measurement of mitral valve area (MVA) and that the mean transmitral gradient, pulmonary artery wedge pressure (PAWP), and pulmonary artery systolic pressure (PP) should also be taken into consideration. †It is controversial as to which patients with less favorable valve morphology should undergo percutaneous mitral balloon valvotomy (PMBV) rather than mitral valve surgery (see text). CXR chest X-ray, ECG electrocardiography, echo echocardiography, LA left atrial, MR mitral regurgitation, MVG mean mitral valve pressure gradient, MVR mitral valve replacement, NYHA New York Heart Association, and 2D 2-dimensional (Reprinted with permission from Bonow et al. [8])
Percutaneous valvulopasty of the mitral valve is accomplished with a single balloon technique which leads to similar results to a double balloon technique with fewer complications [23]. Echocardiography plays a major rule in evaluation of the mitral valve morphology and suitability for mitral balloon valvuloplasty. The Wilkins score (Table 9.2) is a scoring system in which different parts of the mitral valve apparatus are scored depending on mobility, calcification, valve thickening and leaflet thickening with a score less than 9 and less than moderate mitral regurgitation representing a valve favorable for balloon valvotomy [5, 8]. Those patients with mitral valve morphology not favorable for balloon valvotomy can often undergo an open mitral valve comissurotomy or alternatively mitral valve replacement may be needed [5]. If mechanical valve replacement is needed, the choice of valve prosthesis usually depends on the patient’s age, contraindications to chronic anticoagulation as well as patient and surgeon’s preference. Patients with coexisting rheumatic aortic valve disease and severe valve stenosis or regurgitation can be treated with aortic valve replacement.
Aortic Valve Disease
The normal aortic valve consists of three relatively equal semilunar leaflets attached to a fibrous annulus. The right and left coronary arteries arise from the sinuses of Valsalva above the right and left coronary cusps, respectively. The base of the non-coronary cusp is contiguous with the anterior mitral leaflet. Histologically, the leaflets are covered with endothelium and are composed of three distinct layers: the fibrosa composed of circumferentially oriented collagen, the spongiosa that is rich in extracellular matrix (ECM) and valve interstitial cells (myofibroblasts), and the ventricularis comprised of elastic fibers in addition to collagen. The ECM contains proteoglycans and glycosaminoglycans (GAGs).
Aortic Stenosis
Aortic stenosis (AS) is the most common valvular pathology leading to obstruction of left ventricular outflow in women. The etiologies of AS are depicted in the Table 9.3.
Congenital | Acquired |
|---|---|
Unicuspid valve | Calcific |
Bicuspid valve | Trileaflet valve degeneration |
Quadricuspid valve | Post-inflammatory |
Rheumatic < div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|