Chapter 10 Valvular Heart Disease
Severe valvular heart disease imposes a volume or pressure load on the heart that if untreated can result in ventricular impairment and heart failure. Surgical correction prevents further ventricular dysfunction and improves the rate of survival. However, surgery is associated with appreciable morbidity and mortality rates. Therefore, appropriate timing of surgery is essential; excessive delay can lead to irreversible ventricular dysfunction, but if intervention is too early, the risks involved in surgery may outweigh the benefits.
In this chapter the causes, pathophysiology, treatments, and postoperative problems of valvular heart disease are reviewed. Many of the issues relating to comorbid conditions outlined in Chapter 9 apply equally to patients undergoing valve surgery.
SURGICAL TREATMENT OF VALVULAR HEART DISEASE
Valves and Procedures
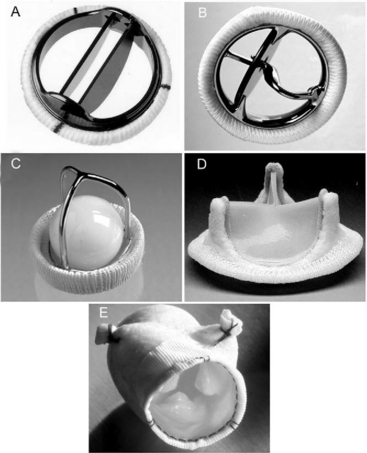
Prosthetic valves may be made of mechanical or bioprosthetic material. Mechanical valves are durable but require lifelong anticoagulation with warfarin. Bioprosthetic valves, at least in the aortic position, do not usually require lifelong warfarin, but are less durable and have a primary failure rate of about 30% at 10 to 15 years.1–3 Bioprosthetic valves are less durable in the mitral position than in the aortic position. In general, mechanical valves are preferred in younger patients, and bioprosthetic valves are preferred in older patients and in patients who may become pregnant. Smaller valves tend to be used in the aortic position (commonly 21 to 25 mm) and larger valves tend to be used in the mitral position (commonly 27 to 31 mm). Mechanical prostheses in each of the four heart valve positions on the frontal and lateral chest radiographs are shown in Fig. 6-6.
Mechanical Valves
Most mechanical valves that are currently implanted are of the bileaflet type (e.g., St. Jude Medical, CarboMedics; Fig, 10-1A). Bileaflet valves have a low profile, provide relatively unobstructed flow, and have minimal areas of stagnation on the downstream side of the disks. Bileaflet valves have a characteristic appearance on echocardiography (Fig. 10-2).
(Reproduced, with permission, from Sidebotham D, Merry A, Legget M: Practical Perioperative Transoesophageal Echocardiography. Fig. 12.2, p. 187. Philadelphia, Butterworth Heinemann, 2003.)
Tilting-disk valves (e.g., Medtronic-Hall; see Fig. 10-1B; Fig. 10-3) have a single pivoting circular disk. Compared to bileaflet valves, tilting-disk valves cause greater impedance to forward flow and a larger area of stagnation on the downstream surface of the disk. The Medtronic-Hall valve has a characteristic central regurgitant jet (see Fig. 10-3).
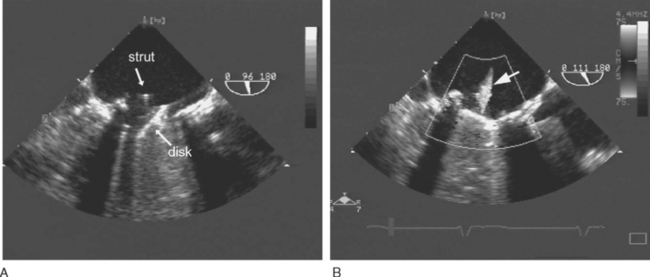
(Reproduced, with permission, from Sidebotham D, Merry A, Legget M: Practical Perioperative Transoesophageal Echocardiography. Fig. 12.4, p. 189. Philadelphia, Butterworth Heinemann, 2003.) TEE, transesophageal echocardiography.
The “ball-in-cage” valve, typified by the Starr-Edwards valve (see Fig. 10-1 C), is the oldest type of mechanical valve. The efficacy of the Starr-Edwards valve is well established; hundreds of thousands of implantations have been performed over 40 years. However, the valve is bulky, results in moderate valvular obstruction, may cause hemolysis, and carries a slightly higher risk for thromboembolism than other valve types. All three types of valves may be used in the mitral or aortic positions.
Bioprosthetic Valves
Bioprosthetic valves may be stented or unstented. Stented valves are made from porcine aortic valves (e.g., Mosaic) or from bovine pericardial tissue (e.g., Carpentier-Edwards Perimount; see Fig. 10-1 D). The stents facilitate implantation and help maintain the three-dimensional structure of the valve. Stented bioprosthetic valves may be used in the aortic or mitral position.
Stentless bioprosthetic valves are made from porcine aortic roots (e.g., Medtronic Freestyle; see Fig. 10-1 E) or cryopreserved human cadaveric aortic roots (homografts). Compared to stented valves, stentless valves provide less obstruction to flow, a reduced risk for endocarditis, and increased durability, but their implantation is more technically demanding, and they can be used only in the aortic and pulmonary positions. Stentless valves may be inserted using a modified subcoronary method as for a standard aortic valve replacement. In addition, some stentless valves, such as the homograft and Freestyle, may be implanted as a freestanding aortic root, in which the aortic valve and sinuses of Valsalva are replaced by the graft, and the coronary arteries are reimplanted as buttons onto fenestrations in the bioprosthesis. An aortic root replacement may be preferred to a subcoronary stentless aortic valve insertion because it can be easier to perform and it provides a larger aortic diameter.
An alternative to a stentless aortic root replacement is the Ross procedure, in which the patient’s own pulmonary valve is transplanted into the aortic position (autograft), and a pulmonary homograft is placed in the pulmonary position. Usually a patient undergoing a Ross procedure (particularly if for aortic incompetence) also requires an aortic root annuloplasty to reduce the annular diameter to between 26 and 28 mm. The pulmonary autograft is durable and may grow with the patient.4 Thus, the Ross procedure is popular for use in younger patients. However, the operation is a long, technically demanding procedure, and it has a mortality rate about twice that of a standard valve replacement. In the long term, autograft dilatation and homograft stenosis can occur.5
SPECIFIC VALVULAR PATHOLOGY
Aortic Stenosis
The causes of aortic valve stenosis are listed in Table 10-1; of these, the most common is calcific degeneration. Calcific degeneration can affect a normal trileaflet valve (Fig. 10-4), a congenitally bicuspid valve, or a bioprosthetic valve. Calcific degeneration of a trileaflet valve typically occurs in patients more than 70 years of age, whereas degeneration of a bileaflet valve typically presents in young adulthood or middle age. Stenosis may also occur at the subvalvular level, either as dynamic left ventricular outflow tract (LVOT) obstruction or secondary to a subaortic membrane.
Table 10-1 Causes of Aortic Valve Stenosis
| Causes | Lesion |
|---|---|
| Calcific degeneration | Predominantly stenosis |
| Degeneration of a bicuspid valve | Stenosis, regurgitation, or mixed |
| Degeneration of a bioprosthetic valve | Stenosis, regurgitation, or mixed |
| Rheumatic aortic valve disease | Stenosis, regurgitation, or mixed |
| Thrombus or pannus formation on a mechanical valve | Stenosis |
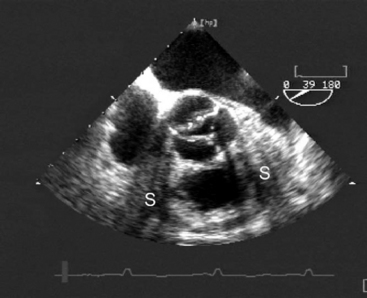
(Reproduced, with permission, from Sidebotham D, Merry A, Legget M: Practical Perioperative Transoesophageal Echocardiography. Fig. 10.3, p. 158. Philadelphia, Butterworth Heinemann, 2003.) TEE, transesophageal echocardiography.
Pathophysiology
Aortic stenosis imposes a pressure load on the left ventricle that results in increased intraventricular pressure and systolic wall tension (Fig. 1-7). Over time, this pressure overload leads to concentric left ventricular hypertrophy, increased end-diastolic pressure and, not infrequently, clinically important diastolic dysfunction. Systolic function is usually preserved until late in the disease process.
Patients with aortic stenosis are at high risk for myocardial ischemia. Myocardial oxygen demands are increased due to the effects of increased pressure work, muscle mass, and wall tension, but coronary blood flow, particularly to the subendocardium, is reduced as a consequence of increased end-diastolic pressure. Also, more than 50% of patients with aortic stenosis have significant coronary artery disease.6 Patients with severe aortic stenosis have a relatively fixed stroke volume and may develop profound hypotension (and myocardial ischemia) in response to modest vasodilation or exercise.
Clinical Features and Investigations
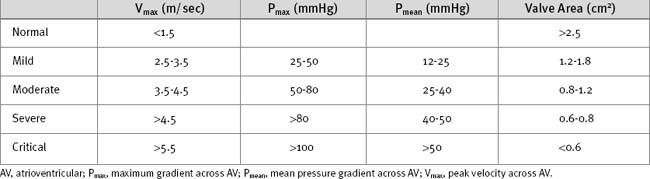
The ECG may demonstrate left ventricular hypertrophy and strain. Nonspecific ST segment changes occur in the majority of patients. Rarely, calcific aortic stenosis is associated with atrioventricular (AV) block. The chest radiograph is frequently normal, although calcification of the valve may be evident. Echocardiography is mandatory to confirm the diagnosis and to assess severity (Table 10-2). Also, it is essential to confirm that the stenosis is due to valvular obstruction and not subvalvular pathology. Significant valvular stenosis is unlikely in the absence of heavy calcification of the valve. Coronary angiography is required in patients over the age of 40 and in those with significant risk factors for coronary artery disease.
Medical Treatment
Symptomatic aortic stenosis is a surgical condition, and medical therapy should not be used to delay surgery. β Blockers provide some relief from angina and may protect against tachyarrhythmias.
Acute Decompensation
Patients with aortic stenosis may present to the intensive care unit with myocardial ischemia, arrhythmias, or congestive cardiac failure. Initial treatment of myocardial ischemia includes oxygen, aspirin, analgesia and, in the absence of heart failure, β blockers. An intraaortic balloon pump (IABP) may be helpful to augment coronary perfusion pressure. In patients with acute coronary syndromes, urgent angiography and combined surgical revascularization and aortic valve replacement should be considered. Pulmonary edema should be treated with diuretics and respiratory support as appropriate. Tachyarrhythmias such as atrial fibrillation are poorly tolerated and must be treated promptly (see Chapter 21). Induction of general anesthesia (e.g., for cardioversion) can cause profound hypotension.
Despite the conventional wisdom that vasodilators are contraindicated in aortic stenosis, low-dose nitroprusside (in slow increments, up to 150 μg/min) has recently been shown to increase cardiac output in normotensive patients with congestive cardiac failure and severe aortic stenosis.7 The authors of this study argue that systemic vasoconstriction may contribute to congestive cardiac failure in patients with severe aortic stenosis. Although this study has received a lot of attention, it must be stressed that potent vasodilators can precipitate cardiovascular collapse in patients with severe aortic stenosis, and that this treatment is not routine.
Surgical Treatment
Sudden death as a result of aortic stenosis is rare in asymptomatic patients. Thus, the presence of symptoms is the primary indication for surgery, irrespective of the aortic valve area or gradient. Recently, levels of B-type natriuretic peptide (BNP; see Chapter 19) have been shown to reflect the onset of symptoms in aortic stenosis. In two studies, patients with symptomatic aortic stenosis had median (± interquartile range) levels of N-BNP (the aminoterminal portion of BNP): 112 pmol/l (70 to 193) and 131 pmol/l (50 to 202) compared to 33 pmol/l (16 to 58) and 31 pmol/l (19 to 56) in asymptomatic patients.8,9 Thus, levels of these hormones may prove to be a useful complement to symptom onset in determining the timing of surgery.
Valve replacement is virtually always required in aortic stenosis because calcified, immobile valve leaflets cannot be repaired. Aortic valve surgery is usually performed through a midline sternotomy. Minimally invasive incisions involving an upper sternotomy and a J incision into the third or fourth intercostal space are also used. The technique of cardiopulmonary bypass (CPB) is similar to that described in Chapter 9. Myocardial protection may be difficult in the setting of severe left ventricular hypertrophy, particularly if there is concomitant aortic regurgitation (see later discussion). Damage to the His bundle may occur during placement of sutures and may cause complete heart block. After CPB, myocardial ischemia may arise as a consequence of (1) a coronary embolus involving aortic or valvular debris; (2) the incorrect seating of a prosthetic valve, causing coronary ostial obstruction; (3) distortion of a coronary artery during reimplantation when performing aortic root replacement. Heavy calcification of the valve or proximal aorta also predisposes to systemic emboli. Percutaneous balloon valvuloplasty of the aortic valve is associated with a very high procedural complication rate; it confers minimal long-term benefit and is rarely performed.10
Postoperative Issues
The operative mortality rate in isolated, first-time aortic valve replacement is 3% to 4%,11,12 but it increases substantially if additional cardiac surgery is required and if left ventricular function is impaired.11 Unlike other valve lesions, the hemodynamic state is immediately improved by valve replacement, even in the presence of preexisting left ventricular dysfunction.
Occasionally, patients with marked left ventricular hypertrophy (particularly involving the basal anterior septum) develop dynamic left ventricular outflow tract (LVOT) obstruction and systolic anterior motion (SAM) of the anterior mitral valve leaflet after aortic valve replacement. Severe SAM can cause hypotension, low cardiac output, and mitral regurgitation. The treatment of this condition is described in Chapter 20.
Aortic Regurgitation
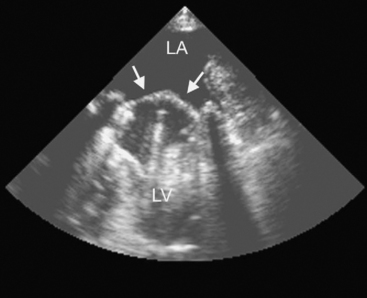
Chronic aortic regurgitation results from aortic root dilatation (Fig. 10-5) or abnormalities of the aortic valve leaflets (Table 10-3). Acute aortic regurgitation results from dissection (see Fig. 11-7), endocarditis and, occasionally, trauma.
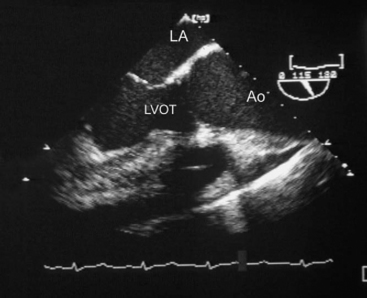
(Reproduced, with permission, from Sidebotham D, Merry A, Legget M: Practical Perioperative Transoesophageal Echocardiography. Fig. 11.4, p. 176. Philadelphia, Butterworth Heinemann, 2003.) Ao, proximal ascending aorta; LA, left atrium; LVOT, left ventricular outflow tract.
Table 10-3 Common Causes of Aortic Regurgitation
| Valvular Dysfunction |
| Rheumatic aortic disease |
| Degeneration of a bicuspid valve |
| Degeneration of a bioprosthetic valve |
| Endocarditis on a native or prosthetic (bioprosthetic or mechanical) valve |
| Leaflet prolapse due to trauma or myxomatous disease |
| Aortic Disease |
| Aortic root dilation |
| Aortic dissection |
Pathophysiology
Because the regurgitant volume is returned to the left ventricle during each diastolic period, aortic regurgitation imposes a volume load on the left ventricle, which results in progressive left ventricular dilatation and eccentric left ventricular hypertrophy. Left ventricular compliance is increased, allowing large ventricular volumes to be accommodated with minimal increase in end-diastolic pressure (see Fig. 1-7). As the left ventricular diameter increases, wall tension and hence afterload are increased. There is compensatory systemic vasodilation. Arterial end-diastolic pressure is low because of diastolic run off into the left ventricle. Thus, the aortic valve opens at a low pressure but peak systolic aortic pressure is increased due to the high stroke volume. Arterial pulse pressure is increased. There may be baroreceptor-mediated tachycardia.
Clinical Features and Investigations
Symptoms occur late in chronic severe aortic regurgitation, by which time important left ventricular dysfunction may have developed. Symptoms, when they do occur, are of congestive cardiac failure: exertional dyspnea and orthopnea. Physical findings include a collapsing pulse, a wide pulse pressure, and diastolic hypotension. The apex beat is displaced laterally and is hyperdynamic. The aortic component of the second heart sound may be soft and there is often a third heart sound. The murmur of aortic regurgitation occurs in early diastole, is high in pitch, and is best heard at the left sternal edge with the patient sitting up and holding his or her breath at expiration.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree