, Domenico Corrado2 and Cristina Basso1
(1)
Cardiovascular Pathology Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padova, Italy
(2)
Cardiology Department of Cardiac, Thoracic and Vascular Sciences, University of Padua, Padova, Italy
6.1 Aortic Valve
6.1.1 Aortic Valve Stenosis
SCD may occur in young people affected by congenital aortic valve stenosis, whether unicuspid or bicuspid with dysplastic cusps [1, 2] (Figs. 6.1, and 6.2). At a young age, the cusps rarely present with a dystrophic calcification, a phenomenon which usually occurs in adult or in the elderly [3–5]. When it happens prematurely, it is usually secondary to endocarditis (Fig. 6.3). SCD is arrhythmic and the arrhythmogenic substrate consists of left ventricular hypertrophy and subendocardial ischemia, in terms of myocytolysis and replacement-type fibrosis (Fig. 6.3) [1, 6].
Subendocardial fibrotic scars are a frequent observation in patients with aortic valve stenosis dying suddenly. The injury is ischemic in nature, since vacuolization of cardiomyocytes and even acute coagulation necrosis are also observed (Fig. 6.3). Clearly, a discrepancy between blood flow demand, due to compensatory left ventricular hypertrophy as a consequence of severe trans-valvular gradient and pressure overload, and coronary artery blood perfusion precipitates ischemic damage, even in the absence of coronary artery disease. An impairment of coronary reserve due to wall stiffness, with constriction of coronary microvasculature during diastole, is an additional factor.
6.1.2 Bicuspid Aortic Valve
A normally functioning bicuspid aortic valve can at risk of SCD. The cardiac arrest is the consequence of spontaneous rupture of the ascending aorta due to dissection, hemopericardium, and cardiac tamponade, because of coexistent aortopathy [1].
The occurrence of aortic dissection in bicuspid aortic valve is five- to sevenfold than the one occurring in normal population with tricuspid aortic valve and nearly 5–6 % of people with aortic dissection show a bicuspid aortic valve. This suggests that bicuspid valve “per se” is a risk factor of aortic dissection, as it is in Marfan syndrome [7, 8].
When bicuspid aortic valve is associated with isthmic aortic coarctation (40–50 % of patients with coarctation present a bicuspid aortic valve), a plausible explanation for the aortic dissection might be the severe hypertension in the prestenotic ascending aorta (Fig. 6.4). However, this is not the case of the dissection occurring in isolated bicuspid aortic valve (Figs. 6.5, and 6.6). A severe disruption of the tunica media, with elastic fragmentation, medial necrosis with loss of smooth muscle cells, and mucoid substance accumulation, is a regular finding at histology [1]. The picture is similar to that observed in Marfan syndrome, the natural history of which is notoriously characterized by early spontaneous aortic dissection by the age of 30–40 years (Figs. 6.7, and 6.8) (see also Chap. 3).
The weakness of tunica media is associated with dilatation of the ascending aorta, which starts prematurely by 15–20 years and then progresses with time up to 4–5 cm in diameter in association with aortic valve incompetence. Aortic dimension of 4.5–5.0 cm is considered the threshold for surgical replacement of the ascending aorta, but we have observed cases of bicuspid aortic valve, aortic dissection, and SCD with much less dilatation.
The atrophy of the elastic fibers of the tunica media accounts for stiffness of the aortic wall with scarce systolic–diastolic excursion, a phenomenon easily detectable at echo and clearly indicating an elastic impairment [9, 10].
The association of bicuspid aortic valve and a degenerative pathology of the aortic root suggest the possible existence of a “bicuspid aortic valve syndrome” in which the bicuspid aortic valve is just one congenital defect in the setting of a developmental pathology of the entire aortic root. Maldevelopment of neural crest, which plays a role in the embryology of both the semilunar valves and the ascending aorta–aortic arch, has been advanced as etiopathogenetic factor.
6.1.3 Supravalvular Aortic Stenosis
It is an autosomal dominant disorder of the elastic fibers, due to deletion of elastin gene at the level of chromosome 7 [12–14]. The lesion is located on the ascending aorta with the shape of a diaphragm, hourglass, or diffuse hypoplasia [12, 13] (Fig. 6.9). It may be either isolated or associated with William’s syndrome, with typical face and intellectual disorders.
The supravalvular stenosis is due to a localized or diffuse intimal fibrous plaque, as a consequence of ventricular systolic ejection on a rigid aorta due to the abnormally stiff elastic tunica media. The coronary ostia may be involved and obstructed.
Cases have been reported of associated anomalies of the aortic valve, the cusps of which may be thickened and fused with the aortic wall. In the latter case, the coronary ostium may be sequestered. In any case, the arrhythmogenic substrate accounting for arrhythmic SCD is an ischemic injury of the myocardium due to ventricular pressure overload, as it is in aortic valve stenosis.
6.2 Mitral Valve
6.2.1 Mitral Valve Prolapse
SCD occurring in young people affected by mitral valve prolapse is arrhythmic, not mechanical [15–17]. Certainly, rupture of chordae tendinae may complicate mitral valve prolapse during natural history and account for abrupt pulmonary edema, but it is rarely a cause of SCD.
In the young, we are dealing with a mitral valve prolapse with coarse deformation of the leaflets, which appear ballooning and thickened due to myxoid changes, in the absence of moderate to severe mitral incompetence and with apparently intact subvalvular apparatus, except for chordal elongation and thinning [17]. The posterior leaflet is usually involved, mostly the medial scallop, well outlined by hooding and mucoid thickening (Figs. 6.10, and 6.11). However, the anterior leaflet may also be affected.
SCD arrhythmic death can be preceded by life-threatening ventricular arrhythmias [17]. The ominous cardiac electrical instability, up to electrical turmoil and cardiac arrest, remains obscure. Several hypothesis have been postulated (endocardial friction of the chordae tendinae, concomitant fatty infiltration of the right ventricle, or specialized conduction system abnormalities), but they are not at all convincing to explain such an abrupt electrical disorder [18–26]. Previous pathology studies in mitral valve prolapse patients dying suddenly mostly focused on mitral valve structural alterations, suggesting a role for leaflets length and thickness, and presence and extent of endocardial plaques. Surprisingly, no investigation did systematically address the left ventricular myocardium to search for the substrate of electrical instability, except for few anecdotic cases. By a thorough histology of the left ventricle, we found evidence of patchy replacement-type fibrosis in the mitral valve papillary muscle as well as in the posterobasal free wall, with or without endocardial plaque, a finding which represents a much more plausible arrhythmogenic substrate, detectable in vivo by cardiac magnetic resonance [17] (Figs. 6.10, and 6.11). In other words, we are dealing with an arrhythmic disorder, which finds an explanation in a myocardial injury associated to mitral valve prolapse. In these circumstances, the term “arrhythmic mitral valve syndrome” is justifiable.
6.3 Tricuspid Valve
6.3.1 Ebstein’s Anomaly
It is well known that patients with Ebstein’s anomaly of the tricuspid valve may present arrhythmias, especially supraventricular tachycardia with ventricular preexcitation in the setting of Wolff–Parkinson–White syndrome [27–30]. The downward displacement of the septal leaflet of the tricuspid valve, which is pathognomonic of the disease, may be associated with accessory pathways (septal “Kent fascicles”), because of the AV ring maldevelopment and direct contact of the atrial with ventricular septal working myocardium, which represents the substrate for AV reentry with onset of supraventricular tachycardia.
There are minor forms of Ebstein’s anomaly, associated with mild tricuspid valve dysfunction, with only minimal downward displacement of septal tricuspid leaflet (so-called microEbstein), but enough to delineate a clear contact between septal atria and ventricular myocardium, as to bypass the specialized AV junction [31] (Fig. 6.12). In these cases, the occurrence of atrial fibrillation even paroxysmal, favored by right atrial dilatation due to tricuspid valve dysfunction, may turn into ventricular fibrillation, due to a short refractory period of the working myocardium of the anomalous AVpathway.
6.4 Image Gallery
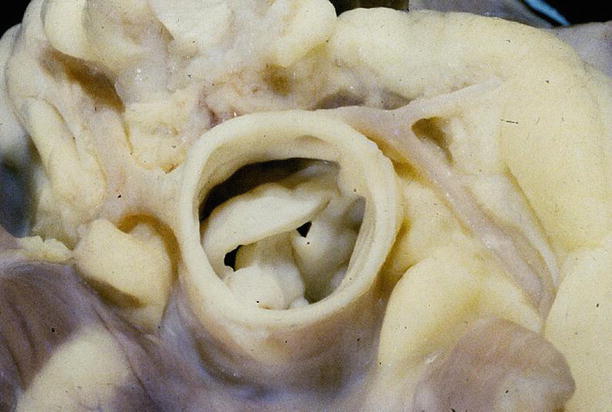
Fig. 6.1
Arrhythmic sudden cardiac death due to aortic valve stenosis in a 9-year-old girl during gymnastic activity. View of the aortic root shows bicuspid aortic valve stenosed by thickened and dysplastic cusps
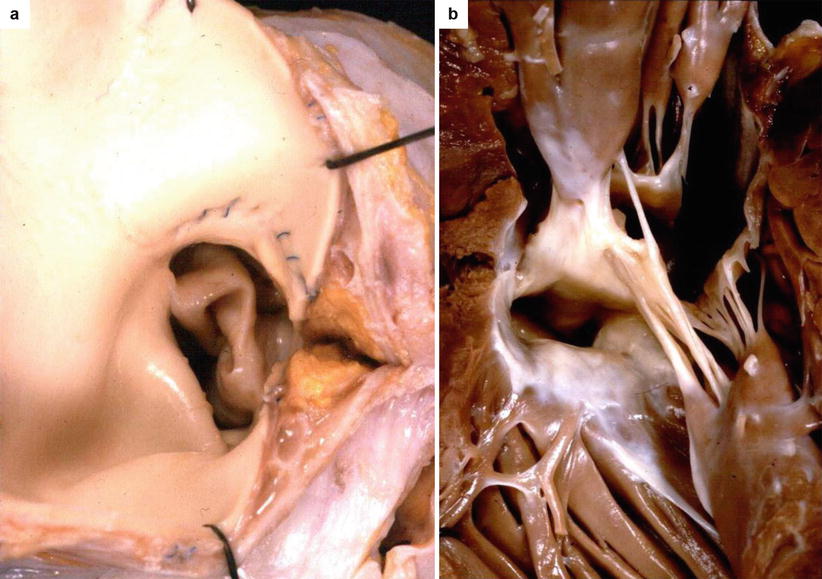
Fig. 6.2
Arrhythmic sudden cardiac death due to aortic valve stenosis in a 17-year-old boy who had a surgical commissurotomy of the aortic valve when he was a child. (a) View of the aortic root shows a stenotic bicuspid aortic valve with thickened and dysplastic cusps: note the previous aortotomy. (a, b) View of the left ventricular outflow tract from below with endocardial subaortic septal plaque
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


