Identification of patients with reversible left ventricular (LV) dysfunction has important prognostic implications after acute myocardial infarction (AMI). This study aimed to determine the value of LV segmental and global longitudinal strains assessed with 3-dimensional (3D) speckle-tracking analysis in predicting improvement of LV function after AMI. One hundred fifty-three patients (80% men, 59 ± 11 years old) with AMI and treated with primary percutaneous coronary intervention underwent 3D echocardiography. LV segmental and global 3D longitudinal strains were assessed with speckle-tracking analysis using a novel dedicated software. At 6-month follow-up, improvement in segmental LV function was defined as a decrease of ≥1 grade in segmental wall motion score. Improvement in global LV function was defined as an absolute improvement ≥5% in LV ejection fraction. Segments with functional improvement at follow-up showed a significantly higher baseline 3D longitudinal strain compared to segments without improvement (−16.4 ± 4.0% vs −7.6 ± 3.5%, p <0.001). A cut-off value of −11.1% for segmental 3D longitudinal strain had 92% sensitivity and 91% specificity in predicting functional improvement. In addition, 67 patients (44%) showed an improvement in global LV function at 6-month follow-up. These patients showed significantly higher baseline global 3D longitudinal strain compared to patients without improvement (−16.7 ± 2.1% vs −13.3 ± 2.6%, p <0.001). Global 3D longitudinal strain provided incremental value over clinical and conventional echocardiographic variables in predicting global LV function improvement (c-statistic improved from 0.64 to 0.71 to 0.84). In conclusion, longitudinal strain assessed by 3D speckle-tracking analysis is an important predictor for segmental and global LV function improvement after AMI.
Echocardiographic measurement of myocardial deformation (strain) by 2-dimensional (2D) speckle-tracking analysis has recently been introduced as a sensitive and accurate tool for quantification of regional and global left ventricular (LV) functions. Initial studies have also shown the value of myocardial deformation parameters for identification at rest of viable myocardium and reversible myocardial dysfunction. The newly developed 3-dimensional (3D) speckle-tracking imaging represents a further advance in myocardial deformation imaging, allowing complete evaluation of all LV segments in the same 3D dataset and overcoming the limitation of 2D out-of-plane speckle motion. However, only a few studies thus far have applied 3D speckle-tracking strain analysis in the clinical setting, and no data exist on its potential role for LV function assessment after acute myocardial infarction (AMI) for regional and global evaluations. Consequently, the present study examined the value of segmental and global longitudinal strains assessed with novel 3D speckle-tracking analysis in predicting LV function improvement after AMI.
Methods
One hundred sixty consecutive patients admitted with first ST-segment elevation AMI were included. All patients underwent coronary angiography and primary percutaneous coronary intervention according to the institutional ST-segment elevation AMI protocol (MISSION!), which is based on the most recent American College of Cardiology/American Heart Association and European Society of Cardiology guidelines. This protocol, designed to improve care around AMI, includes a prehospital, in-hospital, and outpatient clinical framework. The infarct-related artery was determined by electrocardiographic criteria and confirmed during coronary angiography. During percutaneous coronary intervention, final Thrombolysis In Myocardial Infarction flow was assessed. Patient data were prospectively collected in the departmental cardiology information system (EPD-Vision, Leiden University Medical Center, Leiden, the Netherlands) and subsequently analyzed.
Within 48 hours after percutaneous coronary intervention, all patients underwent 2D echocardiography for segmental wall motion evaluation and 3D echocardiography for assessment of LV volumes and LV ejection fraction (LVEF) as part of the routine evaluation of all patients with AMI since the specific imaging software has been available at our institution. Furthermore, 3D speckle-tracking analysis was performed to measure LV segmental and global longitudinal strains.
At 6-month follow-up, 2D and 3D echocardiographic examination were repeated and LV volumes, LVEF, and wall motion were reassessed. Among dysfunctional segments, improvement in segmental LV function was defined as an improvement of ≥1 grade in segmental wall motion score. As previously reported, improvement in global LV function was defined as an absolute improvement ≥5% of 3D LVEF. Analysis of segmental and global LV functions at follow-up was performed blinded to baseline data.
Patients were examined in the left lateral decubitus position with a commercially available ultrasound system (Vivid 7 and E9, GE Healthcare, Horten, Norway) equipped with a 3.5-MHz transducer. Standard 2D images were acquired from parasternal and apical views and digitally stored in cine-loop format. Thereafter image analysis was completed offline with EchoPac 110.0.0 (GE Healthcare). Evaluation of regional wall motion was performed with a 16-segment model as recommended by the American Society of Echocardiography. Each segment was evaluated with a semiquantitative scoring system (1 = normokinetic; 2 = hypokinetic; 3 = akinetic; 4 = dyskinetic), and global wall motion score index was calculated as the average of regional scores.
The 3V phased array transducer was used to obtain the apical 3D full-volume dataset: 6 R-wave-triggered subvolumes acquired for 6 consecutive cardiac cycles during 1 breath-hold were combined to form a larger pyramidal volume including the entire left ventricle. The 3D datasets were digitally stored for off-line quantitative analysis (EchoPac 110.0.0).
Assessment of LV volumes and function was performed with the novel semiautomated quantification algorithm “4D auto LVQ” of EchoPac 110.0.0. The software initially displays apical 4-, 2-, and 3-chamber views and the short-axis view: in each image a manual adjustment of the axes is possible to obtain the best orientation of the 3 apical views and avoid LV foreshortening. After manual identification of the mitral valve plane and the apex with 2 reference points on the end-diastolic and end-systolic frames, the software identifies the endocardial border in each frame for the entire 3D dataset; manual correction of the endocardial border is possible. Subsequently, an LV 3D model is generated and LV volumes and LVEF calculated ( Figure 1 ).
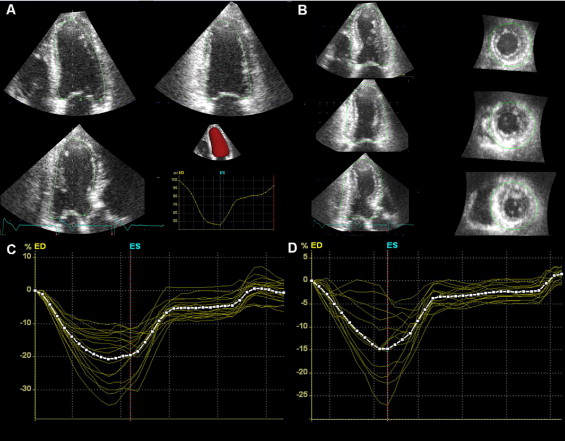
Longitudinal strain measurements were performed in the 3D dataset directly after assessment of LV volumes and function: the novel dedicated software automatically identifies the epicardial border, creating a 3D region of interest, which includes the entire myocardial wall. Myocardial deformation is analyzed by speckle-tracking within the 3D region of interest in 3 different vectors simultaneously ( Figure 1 ). Based on longitudinal strain drift, the software accepts segments with good tracking quality and rejects inadequately tracked segments, allowing the user to manually verify that the region of interest is tracked accurately and rectify the tracking if needed. Results of tracking analysis were exported to a data file, where for each LV segment the observer selected the peak systolic longitudinal strain value. Global 3D longitudinal strain is automatically provided by the software and calculated as the weighted average of the segmental peak longitudinal strain values, where the initial segmental areas are used as weights.
Normal distribution of continuous variables was checked with Kolmogorov–Smirnov 1-sample test and Shapiro–Wilk test, and data were then expressed as mean ± SD. Categorical variables were expressed as absolute numbers and percentages. Differences in continuous variables between 2 groups were evaluated with Student’s t test or Mann–Whitney U test, when appropriate. Differences in categorical variables were assessed with chi-square test. To test intraobserver reproducibility of the 3D LVEF measurement, 2 different datasets acquired during the same echocardiographic study were analyzed by a single observer in 20 randomly selected patients. To test interobserver reproducibility, measurements were repeated by a second experienced observer. Bland–Altman analysis was conducted to assess intra- and interobserver agreements (expressed as absolute value of mean difference ± 2 SD), and intraclass correlation coefficients were calculated. The same analysis was conducted in a different group of 20 randomly selected patients to determine intraobserver and interobserver reproducibilities of 3D strain measurements.
Receiver operator characteristic curve analysis was performed to find the optimal cut-off value of 3D longitudinal strain for predicting segmental functional improvement. The optimal cutoff was defined as the value that provided the highest sensitivity and specificity. Univariate and multivariate linear regression analyses were performed to assess independent determinants of LVEF improvement at follow-up. Variables with a p value <0.1 in univariate analysis were included as covariates in the multivariate model. The incremental value of global 3D longitudinal strain over clinical and conventional echocardiographic variables was evaluated by calculating the chi-square and c-statistic increase of the multivariate model in logistic regression analysis. All statistical tests were 2-sided and a p value <0.05 was regarded as statistically significant. Statistical analysis was performed with SPSS 17.0 (SPSS, Inc., Chicago, Illinois).
Results
Analysis was not feasible (if >3 segments could not be visualized or if the dataset contained visible translation artefacts) in 7 patients (4%) who therefore were excluded from further assessments. Consequently, 153 patients were included in the final analysis.
Intraobserver agreement for 3D LVEF measurements was excellent, with an average difference of 1.1 ± 3.0% (mean ± 2 SD). The intraclass correlation coefficient for intraobserver comparisons was 0.994 (95% confidence interval [CI] 0.985 to 0.998). Interobserver agreement was also excellent, with an average difference of 1.7 ± 3.6% (mean ± 2 SD) and an intraclass correlation coefficient of 0.991 (95% CI 0.978 to 0.997).
Of the 2,448 evaluated segments, 3D speckle-tracking analysis could be performed in 2,174 (89%); 274 (11%) segments were discarded because of inadequate speckle tracking. A good intraobserver agreement for strain measurements was noted, with an average difference of 2.2 ± 5.9% (mean ± 2 SD); the intraclass correlation coefficient was 0.943 (95% CI 0.928 to 0.954). Interobserver agreement was also good, with an average difference of 2.2 ± 6.1% (mean ± 2 SD) and an intraclass correlation coefficient of 0.904 (95% CI 0.864 to 0.933).
Baseline characteristics of the patient population are presented in Table 1 . No patient presented with atrial fibrillation. According to institutional protocol, at baseline all patients were optimally treated with aspirin, clopidogrel, β blockers, angiotensin-converting enzyme inhibitors or angiotensin receptor blockers, and statins, if tolerated ; medical therapy was maintained at 6-month follow-up.
| Characteristics | |
|---|---|
| Age (years) | 59 ± 11 |
| Men/women | 122 (80%)/31 (20%) |
| Body mass index (kg/m 2 ) | 26.6 ± 3.7 |
| Body surface area (m 2 ) | 2.0 ± 0.2 |
| Family history of coronary artery disease ⁎ | 59 (39%) |
| Diabetes mellitus | 16 (11%) |
| Hypertension † | 51 (33%) |
| Smoker | 90 (59%) |
| Hypercholesterolemia ‡ | 25 (16%) |
| Infarct-related coronary artery | |
| Left anterior descending | 69 (45%) |
| Right | 57 (37%) |
| Left circumflex | 27 (18%) |
| Multivessel coronary disease § | 52 (34%) |
| Thrombolysis In Myocardial Infarction flow grade 3 | 129 (84%) |
| Peak troponin T (μg/L) | 4.7 ± 4.8 |
| Left ventricular end-diastolic volume (ml) | 112.3 ± 17.2 |
| Left ventricular end-systolic volume (ml) | 59.7 ± 12.6 |
| Left ventricular ejection fraction (%) | 46.8 ± 6.5 |
| Wall motion score index | 1.8 ± 0.3 |
| Global 3-dimensional longitudinal strain (%) | −14.8 ± 2.9 |
⁎ Defined as evidence of coronary artery disease in a parent or sibling before 60 years of age.
† Blood pressure ≥140/90 mm Hg or previous pharmacologic treatment.
‡ Total cholesterol ≥190 mg/dl or previous pharmacologic treatment.
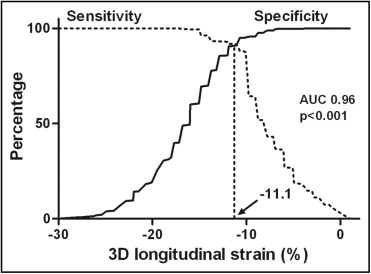
Regarding wall motion analysis, 1,205 (49%) segments had normal wall motion at baseline, whereas 1,243 (51%) segments were classified as dysfunctional, of which 591 (48%) were hypokinetic, 646 (52%) akinetic, and only 6 (0.5%) dyskinetic. At follow-up, wall motion of the dysfunctional segments was reassessed: 596 (48%) segments showed wall motion improvement, whereas 647 (52%) had no wall motion improvement. Among segments with wall motion improvement, 63% were hypokinetic at baseline, whereas 37% were akinetic. Among segments without wall motion improvement, 33% were hypokinetic at baseline, 66% were akinetic, and 1% were dyskinetic. Segments with functional improvement at follow-up showed a significantly higher segmental 3D longitudinal strain at baseline compared to segments without improvement (−16.4 ± 4.0% vs −7.6 ± 3.5%, p <0.001). In receiver operator characteristic curve analysis, a segmental 3D longitudinal strain cut-off value of −11.1% allowed the identification of segments with functional improvement with 92% sensitivity and 91% specificity ( Figure 2 ).
At 6-month follow-up, 67 patients (44%) showed a global LV function improvement (defined as an increase ≥5% in LVEF; of note, test–retest reproducibility of LVEF ± 2 SD did not exceed 4%). Compared to the group of patients without LVEF improvement, no significant differences in baseline clinical characteristics were noted except for peak troponin T level, which was higher in patients without LVEF improvement (p = 0.014; Table 2 ). Among baseline echocardiographic parameters, only wall motion score index (1.7 ± 0.3 vs 1.9 ± 0.3, p = 0.001) and global 3D longitudinal strain (−16.7 ± 2.1% vs −13.3 ± 2.6%, p <0.001) were significantly different in patients with functional LV improvement compared to those without LVEF improvement ( Figure 1 ). At univariate linear regression analysis, global 3D longitudinal strain, wall motion score index, and peak troponin T were significantly related to changes in LVEF. At multivariate linear regression analysis, only global 3D longitudinal strain (beta −0.611, p <0.001) and peak troponin T (beta −0.216, p = 0.003) were independent determinants of LVEF improvement ( Table 3 ). In addition, at multivariate logistic regression analysis, global 3D longitudinal strain provided significant incremental value over clinical and conventional echocardiographic variables in predicting global LV function improvement at follow-up (c-statistic improved from 0.64 to 0.71 to 0.84; Figure 3 ).
| Variable | Improvement in LVEF | p Value | |
|---|---|---|---|
| Yes | No | ||
| (n = 67) | (n = 86) | ||
| Age (years) | 58 ± 11 | 59 ± 10 | 0.545 |
| Men/women | 50 (75%)/17 (25%) | 72 (84%)/14 (16%) | 0.165 |
| Family history of coronary artery disease | 26 (39%) | 33 (38%) | 0.956 |
| Diabetes mellitus | 5 (8%) | 11 (13%) | 0.285 |
| Hypertension | 22 (33%) | 29 (34%) | 0.908 |
| Smoker | 42 (63%) | 48 (56%) | 0.391 |
| Hypercholesterolemia | 15 (22%) | 10 (12%) | 0.074 |
| Infarct-related coronary artery | |||
| Left anterior descending | 26 (39%) | 43 (50%) | 0.167 |
| Right | 29 (43%) | 28 (33%) | 0.173 |
| Left circumflex | 12 (18%) | 15 (17%) | 0.940 |
| Multivessel coronary disease | 21 (31%) | 31 (36%) | 0.542 |
| Thrombolysis In Myocardial Infarction flow grade 3 | 57 (85%) | 69 (80%) | 0.604 |
| Peak troponin T (μg/L) | 3.5 ± 3.3 | 5.8 ± 5.5 | 0.014 |
| Left ventricular end-diastolic volume (ml) | |||
| Baseline | 110.5 ± 15.9 | 113.7 ± 18.2 | 0.263 |
| Follow-up | 101.4 ± 22.2 | 107.3 ± 22.6 | 0.106 |
| Left ventricular end-systolic volume (ml) | |||
| Baseline | 59.0 ± 11.7 | 60.3 ± 13.3 | 0.303 |
| Follow-up | 45.0 ± 13.9 | 58.4 ± 19.8 | <0.001 |
| Left ventricular ejection fraction (%) | |||
| Baseline | 46.2 ± 5.9 | 47.2 ± 6.9 | 0.347 |
| Follow-up | 55.8 ± 7.0 | 46.0 ± 9.8 | <0.001 |
| Wall motion score index | |||
| Baseline | 1.7 ± 0.3 | 1.9 ± 0.3 | 0.001 |
| Follow-up | 1.3 ± 0.3 | 1.6 ± 0.3 | <0.001 |
| Global 3-dimensional longitudinal strain (%) | −16.7 ± 2.1 | −13.3 ± 2.6 | <0.001 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


