The introduction of speckle tracking imaging (STI) allowed the quantification of the regional myocardial function in the right ventricular (RV) free wall using deformation parameters. We sought to evaluate the potential utility of STI at rest and after stress to predict arrhythmogenic RV dysplasia (ARVD). We studied 19 patients with ARVD (diagnosed according to the task force criteria) and 19 healthy age- and gender-matched subjects. Both 2-dimensional and 3-dimensional echocardiography were performed. The RV and left ventricular annular peak systolic velocities were measured using tissue Doppler imaging. The RV-left ventricular peak systolic longitudinal strain (LS) was obtained in the basal, mid, and apical segments in the apical 4-chamber view using STI. An exercise stress-echocardiographic test was undertaken using bicycle ergometry with the patient in the supine position for all patients, and the indexes were assessed at peak effort. The STI measurements were determined using offline analysis programs. The 3-dimensional RV ejection fraction and strain were significantly lower in patients with ARVD than in the controls. The RV strain values at rest did not change significantly during maximum physical effort in the patients with ARVD. The receiver operating characteristic curves suggested that the thresholds offering an adequate compromise between sensitivity and specificity for the detection of ARVD were 9.35 cm/s for the RV annular peak systolic velocity (area under the curve 0.81), 42% for 3-dimensional RV ejection fraction (area under the curve 0.85), −25% for mean global RV-LS (area under the curve 0.86), −18% for the lowest peak systolic RV-LS (area under the curve 0.88), and −1.2 for peak minus baseline global change of stress RV-LS (area under the curve 0.92). In conclusion, STI at rest and during stress might enable quantitative assessment of RV function and the detection of ARVD and have potential clinical value in the treatment of these patients.
Arrhythmogenic right ventricular (RV) dysplasia (ARVD) is a heritable cardiomyopathy characterized by fibrofatty replacement of the RV myocardium, leading to arrhythmias and RV failure and sudden death in young athletes. The diagnosis should be established using a set of criteria as proposed by an international Task Force in 1994 and revised in 2010. Because fat infiltration is seldom the only magnetic resonance imaging abnormality in ARVD and is less sensitive for diagnosis than RV regional dysfunction, and angiocardiography is an invasive technique, which limits its use in follow-up studies, echocardiography has a prominent role in assessing structural and functional changes. The identification of functional abnormalities can result in false-positive data from the visual echocardiographic evaluation, and RV dilation is not a specific finding. The introduction of tissue Doppler imaging and speckle tracking imaging (STI) has allowed the quantification of regional myocardial function in the RV free wall using deformation parameters. However, other conditions that affect regional RV function can be characterized by altered regional deformation in the RV free wall, and changes in regional RV deformation have also been described in endurance athletes. Furthermore, reports of stress testing in patients with ARVD have been scanty, and the behavior of the strain parameters after stress has not been reported. Accordingly, we sought to evaluate the potential utility of STI at rest and after stress to predict ARVD and to determine its potential role compared to conventional and 3-dimensional echocardiographic parameters.
Methods
We studied 19 patients with ARVD diagnosed in accordance with the task force criteria. All patients underwent a detailed history and physical examination, 12-lead electrocardiography, conventional and strain echocardiography, Holter electrocardiography, and stress testing. Electrophysiology, ventricular angiography, and/or magnetic resonance imaging (in patients without defibrillators) were performed in patients with equivocal findings. In none of the patients were symptoms of right-sided heart failure present. Antiarrhythmic drug therapy was not discontinued. All patients were in stable sinus rhythm during the echocardiographic examination. A total of 12 patients underwent coronary angiography, with negative findings. A total of 19 healthy age- and gender-matched adults were selected as the control group. All subjects provided written informed consent.
All patients underwent transthoracic echocardiography with a commercially available ultrasound system (Vivid E9, General Electric, Horten, Norway). Established criteria were used to measure the right chambers. Immediately after 2-dimensional echocardiography, the patients underwent 3-dimensional echocardiography, which was performed as a full-volume scan of the left ventricular (LV) and RV from the apical position. The images were stored digitally for offline analysis using TomTec software (TomTec Imaging Systems, Unterschleissheim, Germany). The methods and accuracy of the measurements of end-systolic and end-diastolic RV volumes have been previously reported. The process of volume determination was done 2 times for each patient. The papillary muscles were not included in the volume estimation.
The general principles that underlie the STI modalities have been previously reported. The LV longitudinal strain (LVLS) was defined as the average of the negative LS of 6 segments of the septal and lateral walls on the apical 4-chamber view. The assessment of LV rotation by 2-dimensional speckle tracking strain imaging required the acquisition of the LV short axis at the basal and apical levels. The basal level was defined as that showing the mitral valve tip and the apical level as that just proximal to the level with LV cavity obliteration at end-systole. LV torsion or twist was defined as the net difference (in degrees) of the apical and basal rotation at the isochronal points. Normalized torsion was defined as torsion divided by the LV ventricular diastolic longitudinal length between the apex and mitral planes. The peak diastolic untwisting velocity and interval to peak untwisting velocity were measured.
Special care was taken to ensure an adequate field of view to image the entire right ventricle by narrowing the 2-dimensional sector width or positioning the transducer down an intercostal space and laterally. To assess the regional and global RV systolic function in the longitudinal direction ( Figure 1 ), we adopted a 6-segment RV model (basal RV lateral wall, mid-RV lateral wall, apical RV wall, apical septum, mid-septum, and basal septum). The lowest peak systolic strain in all 3 segments of the RV free wall was determined, as recently reported. Global strain was calculated by averaging the local strain values along the entire right ventricle. Reduced strain is indicated by less negative values throughout the report. The echocardiograms were analyzed by an echocardiologist who was unaware of the clinical data using dedicated software (EchoPAC BT11, General Electric, Horten, Norway).
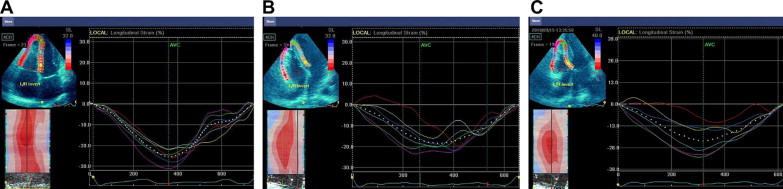
A symptom-limited or submaximum (≤85% of the age-predicted maximum heart rate) exercise stress-echocardiographic test was undertaken using bicycle ergometry with the subject in the supine position, with an initial workload set at 25 W and 25-W increments every 2 minutes. The heart rate and rhythm were continuously recorded using 12-lead electrocardiography, and the blood pressure was measured manually during the last 30 seconds of each stage by sphygmomanometry. Exercise testing was interrupted promptly in the case of significant ventricular arrhythmia, limiting breathlessness, dizziness, muscular exhaustion, chest pain, or severe systemic hypertension. The functional and echocardiographic indexes were assessed at peak effort. The strain parameters after stress testing were compared to those from 19 healthy selected controls and 19 healthy age- and gender-matched endurance athletes.
The data are presented as the mean ± SD. The variables were compared between groups using Student’s t test. Differences were considered statistically significant when p <0.05. STI diagnostic accuracy was analyzed using receiver operating characteristic curves, with ≥4 points in the task force criteria (major criteria, 2 points; minor, 1 point) as the reference standard.
Results
Although great care was taken to ensure the quality of the data collected, 19.9% of all wall segments had to be excluded from the analysis because the strain traces were defined as noninterpretable. Of the 22 initially evaluated patients with ARVD, 19 were included in the present study. The overall feasibility of speckle tracking echocardiography at rest and peak stress was 86%. The percentage of variability was <10% for the at rest parameters and ≤18% for the data obtained during exercise.
Of the 19 patients, 8 had a history of syncope and/or palpitations. Ventricular tachycardia of left bundle branch block morphology was confirmed in 6 of these patients and supraventricular tachycardia in 2. At the evaluation, 6 patients were taking sotalol, 2 were taking metoprolol, 1 was taking metoprolol and sotalol, and 1 was taking amiodarone and metoprolol. Finally, 7 patients had had an automatic cardioverter defibrillator implanted.
Patients with ARVD had a decreased 3-dimensional RV ejection fraction compared to the normal controls ( Tables 1 and 2 ). In the ARVD group, analysis of regional deformation showed that the strain values were significantly reduced in all 3 segments in the RV free wall compared to those in the controls ( Table 2 ). No significant changes for any of the measured deformation parameters were observed in the 10 patients receiving antiarrhythmic medications compared to patients not receiving therapy, although a trend was seen toward lower values in patients receiving medication. The lowest RV strain ( Tables 1 and 2 ) was abnormal (<−18%) in 13 of 19 patients with ARVD (68%) and 1 of 19 controls (5%; p <0.0005). The global RV strain was reduced (i.e., less negative than −25%) in 12 of 19 patients (61%) and 2 of 12 normal controls (12%; p <0.001). The LV parameters showed a reduction of the LVLS (−19.6 ± 2.1% vs −20.9 ± 2.4%, p <0.05) and increased LV torsion (18.7° ± 4.5° vs 14.6° ± 4.1°, p <0.05). The global LV strain was reduced (<−25%) in 38% of patients and 9% of normal controls (p <0.01).
| Gender | Age (yrs) | RVOT Plax (mm/m 2 ) | RVFAC (%) | TAPSE (mm) | TV S a (cm/s) | 3D-RVEF (%) | Global RVLS (%) | Lowest RVLS (%) | Stress Global RVLS (%) |
|---|---|---|---|---|---|---|---|---|---|
| Male | 21 | 14.2 | 53 | 22.3 | 13.5 | 61 | −27.2 | −19.2 | −30.1 |
| Female | 23 | 15.1 | 57 | 26.1 | 14.1 | 64 | −31.1 | −22.3 | −33.6 |
| Male | 28 | 14.2 | 53 | 20.6 | 12.1 | 59 | −31.8 | −24.7 | −35.2 |
| Female | 29 | 15.7 | 52 | 23.2 | 10.4 | 57 | −30.2 | −25.1 | −33.3 |
| Male | 32 | 13.1 | 49 | 21.3 | 10.8 | 55 | −29.7 | −22.4 | −31.4 |
| Male | 33 | 12.4 | 50 | 24.7 | 15.1 | 52 | −24.1 | −21.2 | −31.5 |
| Male | 36 | 11.2 | 45 | 25.8 | 13.7 | 49 | −25.7 | −22.3 | −30.7 |
| Female | 37 | 12.7 | 54 | 24.2 | 12.2 | 55 | −28.3 | −24.4 | −32.8 |
| Female | 38 | 17.1 | 52 | 26.1 | 14.6 | 63 | −33.1 | −28.2 | −36.1 |
| Male | 41 | 12.8 | 50 | 21.6 | 11.9 | 53 | −28.7 | −23.2 | −32.2 |
| Male | 43 | 12.8 | 46 | 19.7 | 11.1 | 54 | −24.2 | −20.1 | −28.7 |
| Female | 45 | 14.7 | 55 | 25.1 | 14.1 | 58 | −30.8 | −20.6 | −33.2 |
| Male | 50 | 14.1 | 48 | 20.7 | 13.4 | 61 | −29.3 | −22.1 | −32.1 |
| Male | 51 | 13.1 | 46 | 22.2 | 12.6 | 55 | −25.7 | −21.3 | −32.2 |
| Female | 53 | 12.8 | 52 | 25.5 | 13.2 | 54 | −31.1 | −19.2 | −34.1 |
| Male | 55 | 15.2 | 45 | 20.3 | 12.9 | 56 | −30.2 | −20.2 | −32.9 |
| Male | 60 | 16.2 | 42 | 19.7 | 14.5 | 51 | −17.8 | −17.2 | −25.2 |
| Female | 61 | 14.6 | 53 | 24.8 | 13.8 | 58 | −29.8 | −23.3 | −33.2 |
| Male | 67 | 13.1 | 48 | 24.1 | 11.7 | 60 | −29.1 | −22.5 | −33.1 |
| Mean ± SD | 42.2 ± 13.2 | 13.9 ± 1.4 | 49.9 ± 3.9 | 23.1 ± 2.3 | 12.9 ± 1.4 | 57.1 ± 4.3 | −28.6 ± 2.8 | −22.7 ± 2.3 | −32.2 ± 2.4 |
| Gender | Age (yrs) | RVOT Plax (mm/m 2 ) | RVFAC (%) | TAPSE (mm) | TV S a (cm/s) | 3D-RVEF (%) | Global RVLS (%) | Lowest RVLS (%) | Stress Global RVLS (%) |
|---|---|---|---|---|---|---|---|---|---|
| Male | 22 | 21.2 | 43 | 17.8 | 10.2 | 47 | −20.1 | −15.1 | −21.3 |
| Female | 23 | 18.6 | 46 | 18.7 | 12.1 | 48 | −22.4 | −12.4 | −24.4 |
| Male | 27 | 15.3 | 41 | 21.9 | 8.4 | 41 | −19.7 | −8.1 | −21.6 |
| Female | 29 | 17.8 | 47 | 17.2 | 9.1 | 51 | −25.1 | −19.4 | −27.2 |
| Male | 30 | 20.1 | 40 | 18.3 | 8.7 | 42 | −18.7 | −8.3 | −19.8 |
| Male | 31 | 22.4 | 48 | 17.2 | 11.2 | 50 | −16.1 | −12.5 | −17.9 |
| Male | 36 | 23.2 | 43 | 23.5 | 9.4 | 43 | −16.2 | −11.6 | −17.4 |
| Female | 38 | 19.5 | 44 | 16.4 | 8.2 | 41 | −16.4 | −7.2 | −18.8 |
| Female | 39 | 16.2 | 52 | 20.6 | 11.2 | 55 | −25.5 | −19.1 | −27.4 |
| Male | 40 | 21.2 | 41 | 17.7 | 8.6 | 44 | −25.2 | −19.3 | −25.5 |
| Male | 43 | 22.3 | 43 | 17.2 | 9.6 | 45 | −15.1 | −8.2 | −15.9 |
| Female | 46 | 20.2 | 47 | 15.8 | 9.1 | 48 | −17.2 | −10.1 | −18.9 |
| Male | 48 | 25.2 | 39 | 16.9 | 8.2 | 40 | −17.1 | −9.2 | −18.8 |
| Male | 50 | 15.6 | 48 | 18.1 | 12.2 | 52 | −25.3 | −18.1 | −25.9 |
| Female | 51 | 23.2 | 42 | 18.6 | 7.7 | 42 | −25.2 | −19.2 | −26.8 |
| Male | 56 | 18.3 | 39 | 20.2 | 9.4 | 40 | −14.1 | −7.7 | −15.5 |
| Male | 57 | 15.4 | 35 | 18.4 | 8.5 | 40 | −25.7 | −18.2 | −27.1 |
| Female | 63 | 16.8 | 44 | 20.5 | 14.7 | 44 | −17.9 | −8.1 | −18.8 |
| Male | 67 | 23.5 | 36 | 19.7 | 10.1 | 39 | −25.2 | −11.3 | −25.8 |
| Mean ± SD | 41.9 ± 13.2 | 19.8 ± 3.1 ∗ | 43.1 ± 4.3 ∗ | 18.6 ± 1.9 ∗ | 9.8 ± 1.8 ∗ | 44.8 ± 4.7 † | −20.4 ± 4.2 ‡ | −12.7 ± 4.6 ‡ | −21.3 ± 4.2 § |
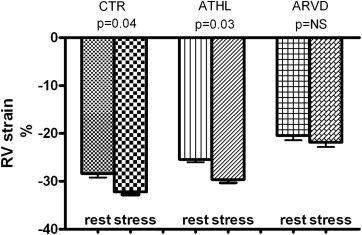
Of the 19 patients, 8 did not present with any arrhythmia during or after exercise, 9 showed sporadic ventricular premature beats at peak exercise and immediately after, 1 showed multiple ventricular premature beats, and 1 experienced a short ventricular tachycardia consisting of 9 consecutive ventricular premature beats near peak exercise. None of the patients had signs of myocardial ischemia on the exercise electrocardiogram. The heart rate increment and workload were greater in the controls than in the patients with ARVD (p = 0.04). In the normal subjects, the RV strain values increased significantly during exercise ( Table 2 and Figure 2 ). In the endurance athletes ( Figure 2 ), the RV strain values were decreased at rest compared to those of the normal controls (−25.4 ± 2.5% vs −28.6 ± 2.7, p <0.01) and increased during exercise (at rest −25.4 ± 2.5%, exercise −29.6 ± 2.9%, p = 0.03). In the patients with ARVD, the decreased RV strain values found at rest did not change significantly during maximum physical effort ( Table 2 and Figures 1 and 2 ). The global longitudinal LV strain and torsion did not have significant changes with exercise. The exclusion of the 7 patients who did not have coronary angiographic confirmation did not significantly affect the results.