Some patients diagnosed with arrhythmogenic right ventricular cardiomyopathy (ARVC) are eventually found to have cardiac sarcoidosis (CS). Accurate differentiation between these 2 conditions has implications for immunosuppressive therapy and familial screening. We sought to determine whether cardiac magnetic resonance imaging (MRI) could be used to identify the characteristic findings to accurately differentiate between CS and ARVC. Consecutive patients with a diagnostic MRI scan indicating CS and/or ARVC constituted the cohort. All patients diagnosed with CS had histologic confirmation of sarcoidosis, and all patients with ARVC met the diagnostic task force criteria. The cardiac MRI data were retrospectively analyzed to identify possible differentiating characteristics. Of the patients, 40 had CS and 21 had ARVC. Those with CS were older and had more left ventricular scar. The presence of mediastinal lymphadenopathy or left ventricular septal involvement was seen exclusively in the patients with CS (p <0.001). A family history of sudden cardiac death was seen only in the ARVC group (p = 0.012). The right ventricular ejection fraction and ventricular volumes were also significantly different between the 2 groups. In conclusion, patients with CS have significantly different cardiac MRI characteristics than patients with ARVC. The cardiac volume, in addition to the degree and location of cardiac involvement, can be used to distinguish between these 2 disease entities. The presence of mediastinal lymphadenopathy and left ventricular septal scar favors a diagnosis of CS and not ARVC. Consideration of CS should be given if these MRI findings are observed during the evaluation for possible ARVC.
The accurate diagnosis of arrhythmogenic right ventricular (RV) cardiomyopathy (ARVC) is important for familial genetic screening and the prevention of sudden death. Similarly, the early identification of cardiac sarcoidosis (CS) will lead to the initiation of immunosuppressive therapy, possibly preventing subsequent progression to heart failure and arrhythmia. Given the overlap in clinical presentation and diagnostic criteria, the high false-negative rate of the reference standard, endomyocardial biopsy, and the importance of the correct diagnosis for patient care, a tool for the accurate differentiation of these 2 conditions is needed. Cardiac magnetic resonance imaging (MRI) is frequently used as a part of the cardiac workup for both of these conditions and can be used to accurately identify the distribution of scar patterns, scar characterization, and measure the ventricular chamber size and function. The identification of MRI characteristics able to differentiate CS from ARVC could prompt additional clinical testing and eventually lead to a better and more accurate diagnosis approach. Therefore, we sought to investigate a series of patients with CS and ARVC to evaluate the utility of cardiac MRI criteria as a tool in the differential diagnosis.
Methods
The study cohort consisted of 61 consecutive patients referred to our institutions for evaluation of the presence of CS or ARVC. Of the patients, 38% of those with ARVC and all of those with CS underwent workup at the University of Colorado. From March 2004 to December 2009, an active screening program was underway for CS in selected patients with biopsy-proven pulmonary sarcoidosis at the University of Colorado Hospital. Patients with extracardiac sarcoidosis and signs or symptoms that could indicate early cardiac involvement were screened. ARVC was diagnosed when a combination of major and minor diagnostic criteria met the task force definition set forth in 1994 and updated in 2010. The baseline patient characteristics, including diabetes, hypertension, hyperlipidemia, and chronic kidney disease, were identified by chart review.
Cardiac MRI was performed according to the standard imaging protocols using a 1.5 Tesla system, dedicated cardiac phased-array coils, and electrocardiographic triggering. Axial, coronal, and sagittal scouts were first obtained. Axial T 1 -weighted imaging was followed by T 2 -weighted dark blood images from the pulmonary artery to the inferior aspect of the heart. Reconstructed, 3-dimensional, delayed gadolinium contrast enhancement with review of selected 2-dimensional images to confirm relevant findings and short-tau inversion-recovery imaging were routinely acquired. The right ventricle and RV outflow tract were included in all algorithms. Perpendicular, vertical, horizontal, and short-axis images were obtained using single breath-hold imaging with a steady-state precession electrocardiographic-gated cine technique to qualify and quantify regional myocardial function. Chamber volumes and ejection fractions were calculated using computerized algorithms. RV volumetric quantification and structural assessment was routinely performed. Double inversion recovery nulling the myocardium and individually calculated inversion times were performed before delayed contrast imaging. The MRI data were interpreted by cardiologists and radiologists with expertise in cardiac imaging. The ongoing clinical workup and diagnosis were available to the MRI reader.
Data are presented as the mean ± SD or as absolute numbers with rounded percentages. Comparisons between groups were made using the unpaired t test for continuous variables or Fisher’s exact test for dichotomous nominal variables. The sensitivity, specificity, positive and negative predictive values, and positive and negative likelihood ratios were calculated using standard formulas. A p value of <0.05 was considered statistically significant.
Results
The clinical characteristics of the 40 patients with CS and 21 with ARVC are listed in Table 1 . Approximately 2/3 of both cohorts were men. The patients in the CS group were older and more likely to have been diagnosed with hypertension. A family history of sudden cardiac death alone (p = 0.012) and a family history of sudden cardiac death or ARVC (p <0.001) strongly distinguished these 2 cohorts. No patient in the CS group reported a history of sudden cardiac death. Most MRI characteristics were significantly different between the ARVC and CS groups ( Table 2 ). Those with ARVC had lower left ventricular (LV) ejection fractions and increased LV diastolic and systolic volumes. The patients with ARVC also had lower RV ejection fractions and increased RV diastolic and systolic volumes. Isolated LV involvement was demonstrated only in those with CS. Every patient with ARVC had RV involvement; however, only 48% of those with CS had RV involvement. The presence of mediastinal lymphadenopathy was seen only in the CS group and was present in 68% of the patients (p <0.001; Figure 1 ).
| Characteristic | CS (n = 40) | ARVC (n = 21) | p Value |
|---|---|---|---|
| Age (years) | 56 ± 12 | 44 ± 12 | <0.001 |
| Coronary artery disease | 3 (8%) | 1 (5%) | 1.00 |
| Diabetes mellitus | 7 (18%) | 1 (5%) | 0.243 |
| Hypertension | 18 (45%) | 2 (10%) | 0.009 |
| Hyperlipidemia | 10 (25%) | 5 (24%) | 1.00 |
| Chronic kidney disease | 3 (8%) | 0 | 0.545 |
| Men | 25 (63%) | 14 (67%) | 0.787 |
| Family history of sudden cardiac death | 0 | 4 (19%) | 0.012 |
| Family history of sudden cardiac death or arrhythmogenic right ventricular cardiomyopathy | 0 | 13 (62%) | <0.001 |
| Characteristic | CS (n = 40) | ARVC (n = 21) | p Value |
|---|---|---|---|
| Left ventricular ejection fraction (%) | 66 ± 14 | 55 ± 10 | 0.073 |
| Left ventricular mass (g) | 129 ± 18 | 130 ± 38 (n = 8) | 0.425 |
| Left ventricular systolic volume (cm 3 ) | 36 ± 16 | 72 ± 31 | <0.001 |
| Left ventricular diastolic volume (cm 3 ) | 103 ± 3 | 155 ± 42 | <0.001 |
| Right ventricular ejection fraction (%) | 49 ± 11 | 40 ± 9 | 0.017 |
| Right ventricular mass (g) | 78 ± 30 | 80 ± 34 (n = 6) | 0.618 |
| Right ventricular systolic volume (cm 3 ) | 62 ± 29 | 104 ± 42 | 0.002 |
| Right ventricular diastolic volume (cm 3 ) | 124 ± 44 | 176 ± 63 | 0.004 |
| Delayed enhancement | 29 (73%) | 4 (19%) | <0.001 |
| Short inversion time inversion-recovery | 16 (40%) | 3 (14%) | 0.047 |
| Right ventricular involvement | 19 (48%) | 21 (100%) | 0.005 |
| Isolated right ventricular involvement | 8 (20%) | 16 (76%) | <0.001 |
| Left ventricular involvement | 25 (63%) | 5 (24%) | 0.004 |
| Isolated left ventricular involvement | 21 (53%) | 0 | <0.001 |
| Mediastinal lymphadenopathy | 27 (68%) | 0 | <0.001 |
| Septal involvement | 17 (43%) | 0 | 0.001 |
| Septal or mediastinal lymphadenopathy or isolated left ventricular involvement | 38 (95%) | 0 | <0.001 |
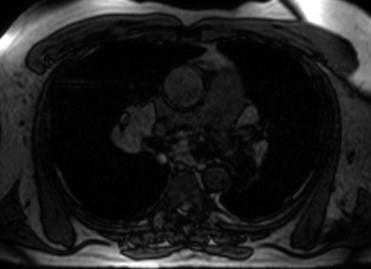
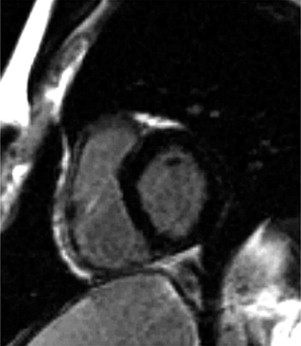
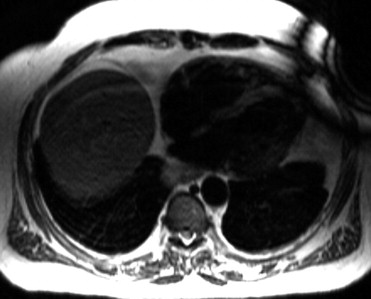
Delayed contrast enhancement was seen in 73% of the CS and 19% of the ARVC groups ( Figures 2 and 3 ). The scar location was different between the 2 cohorts. Isolated LV involvement was seen only in the CS group. Forty-three percent of the CS group and none of the ARVC group demonstrated intraventricular septum dysfunction or delayed contrast enhancement of the septum. In fact, 78% of those with CS had septal involvement or mediastinal lymphadenopathy and none of those with ARVC had evidence of either on the MRI scan. The presence of short-tau inversion-recovery positivity was found more often in the CS group, likely indicating that some patients with CS had an acute or subacute inflammatory response. Because isolated LV involvement, the presence of mediastinal lymphadenopathy, and septal involvement were exclusive to the CS group, the specificity for each individually was 1.0; therefore, receiver operating characteristic curves were not calculated. The individual sensitivity for the MRI findings for isolated LV involvement, mediastinal lymphadenopathy, and septal involvement was 0.53, 0.68, and 0.43, respectively ( Table 3 ). Not having any of these MRI characteristics had a sensitivity of 0.95 and a negative predictive value of 0.91 for ruling out CS. The negative likelihood ratio of not having isolated LV involvement, mediastinal lymphadenopathy, or septal involvement was 0.05.
| Variable | Sensitivity (%) | Specificity (%) |
|---|---|---|
| Isolated left ventricular involvement | 53% | 53% |
| Mediastinal lymphadenopathy | 68% | 62% |
| Septal involvement | 43% | 48% |
| Septal involvement or mediastinal lymphadenopathy or isolated left ventricular involvement | 95% | 91% |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


