The objective of this study was to investigate the systolic to diastolic duration ratio (S:D ratio) in children with pulmonary arterial hypertension (PAH) and its association with right ventricular (RV) performance, hemodynamics, 6-minute walk test, clinical outcomes, and survival. We reviewed 503 serial echocardiograms in 47 children with PAH (mean pulmonary artery pressure ≥25 mm Hg) and compared the S:D ratio, assessed from Doppler flow of tricuspid valve regurgitation, to that in 47 age-matched controls. We reviewed echocardiograms, catheterization data, 6-minute walk tests, clinical data, lung transplantation, and death and used univariate linear regression models with a maximum likelihood algorithm for parameter estimation to investigate associations between S:D ratio and RV function, hemodynamics, functional capacity, and clinical outcomes. The S:D ratio was significantly higher in patients than in controls (1.38 ± 0.61 vs 0.72 ± 0.16, p <0.001). A higher S:D ratio was associated with worse echocardiographic RV fractional area of change, worse catheterization hemodynamics, shorter 6-minute walk distance, and worse clinical outcomes independent of pulmonary resistance or pressures. An increase of 0.1 in the S:D ratio was associated with a 13% increase in yearly risk for lung transplantation or death (hazard ratio 1.13, p <0.001). An S:D ratio 1.00 to 1.40 was associated with a moderate risk and an S:D ratio >1.40 was associated with a high risk of a negative outcome. In conclusion, in children with PAH, an increased S:D ratio is temporally associated with worse RV function, hemodynamics, exercise capability, clinical status, and survival.
Right ventricular (RV) failure is the main cause of clinical symptoms, exercise intolerance, and eventually death in children with pulmonary arterial hypertension (PAH). Echocardiography is used to estimate pulmonary arterial pressures and evaluate disease progression, response to therapy, and RV adaptation to increased afterload. However, assessment of RV function is difficult and noninvasive measurements of RV performance are needed. We previously showed that the ratio between the duration of systole and diastole (S:D ratio), a fundamental characteristic of cardiac action, is an index of global left ventricular function in pediatric cardiomyopathy and of RV function in hypoplastic left heart syndrome. However, whether the S:D ratio is an indicator of RV performance in the face of increased pulmonary pressures has not been studied. We hypothesized that the S:D ratio is abnormally increased in children with increased pulmonary arterial pressures, whether associated with congenital heart disease or with idiopathic pulmonary arterial hypertension, and that a high S:D ratio is associated with worse exercise tolerance and adverse survival in this population. The objectives of this study were to investigate the S:D ratio in children with PAH, determine its association with hemodynamics and exercise tolerance, and determine its association with clinical outcomes.
Methods
PAH was defined according to World Health Organization criteria as a mean pulmonary arterial pressure ≥25 mm Hg at rest (measured at cardiac catheterization). Patients with idiopathic PAH or PAH related to repaired or unrepaired intracardiac shunts and/or patent ductus arteriosus were retrospectively identified through the cardiology electronic database at our institution. We included patients from a 10-year period from January 1999 to December 2008 who had undergone a 6-minute walk test, cardiac catheterization, and echocardiography within a 30-day period. We excluded patients with an absent or inadequate tricuspid regurgitation (TR) Doppler tracing, single-ventricle physiology, or transient neonatal pulmonary hypertension. Patients too young to perform a 6-minute walk test but who were otherwise eligible were included. Age-matched healthy children who underwent echocardiography for evaluation of a functional murmur (n = 24) or chest pain (n = 23) and in whom echocardiogram was normal served as controls.
Echocardiography was performed on an IE-33 (Philips Ultrasound, Bothell, Washington), an HDI 5000 CV (ATL Ultrasound, Bothell, Washington), or a Vivid 7 (GE, Horten, Norway) system with transducer frequencies appropriate for patient size. Measurements were made off-line from digitally stored images using commercially available software (Syngo Dynamics, Siemens, Malvern, Pennsylvania) and from videotapes using a Vivid 7 ultrasound system. One cardiac cycle to 3 consecutive cardiac cycles (based on availability) were analyzed, and results averaged. Echocardiographic measurements were performed by a single investigator (JA). Durations of systole and diastole were measured from the clearest Doppler signal of TR from the apical (most common) or parasternal view. Systolic duration was measured as duration (onset to termination) of TR. Diastolic duration was measured from termination of TR to onset of the subsequent TR tracing. The ratio between the systolic and diastolic duration was then calculated from these intervals ( Figure 1 ).
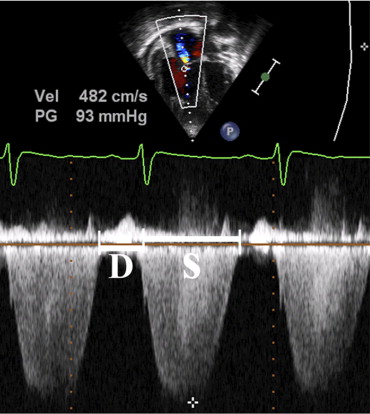
Qualitative subjective evaluation of RV systolic function was classified as normal, mildly decreased, moderately decreased, or severely decreased as determined by the interpreting echocardiographer at time of clinical echocardiography. RV end-diastolic dimension was recorded by M-mode from the parasternal long-axis view at the level of the mitral valve leaflet tips and the Z-score, based on institutional normal data, reported. RV fractional area of change (FAC; percentage) was calculated anew from the apical 4-chamber view as percent FAC ([end-diastolic area − end-systolic area]/end-diastolic area). RV systolic pressure was calculated from the TR Doppler by the modified Bernoulli equation. An assumed right atrial pressure of 5 mm Hg was added to this result. Mean pulmonary artery pressure was estimated from the early velocity of the pulmonary regurgitation jet.
Baseline RV systolic and diastolic pressures; systolic, diastolic, and mean pulmonary artery pressures; systolic and diastolic systemic blood pressures; pulmonary blood flow indexed for body surface area (indexed pulmonary blood flow), systemic blood flow indexed for body surface area, pulmonary vascular resistance indexed for body surface area, and changes in pulmonary vascular resistance indexed for body surface area with exposure to 100% oxygen; 40 ppm of inhaled nitric oxide, sildenafil given by nasogastric tube, and nebulized prostacyclin were recorded. The distance walked in 6 minutes (meters) and pulse oxymetry at baseline, at peak exercise, and at recovery were recorded.
Clinical worsening, based on World Health Organization functional guidelines, was defined as new or worsening shortness of breath, history of progressive exercise limitation, chest pain, presyncope, or syncope on exertion. New York Heart Association class was also recorded. Lung transplantation and death were obtained from the medical record.
Data were recorded for each patient at 4 predefined nonoverlapping points: (1) initial evaluation at diagnosis, (2) 6 to 9 months after starting therapy to assess response, (3) intermediate follow-up after catheterization and echocardiography (after these tests were obtained due to clinical change or as an intermediate point between initiation of therapy and the most recent evaluation), and (4) the most recent evaluation. In addition, to determine associations between the S:D ratio and clinical outcomes, we recorded the duration of systole and diastole and S:D ratio on every echocardiogram obtained in every patient since diagnosis. Age at diagnosis obtained from clinical records and age at first available echocardiogram were recorded and reported separately (these were not synonymous).
Data are presented as means ± SDs, medians with minimum and maximum values, and frequencies as appropriate. Paired t tests were used to determine the difference in S:D ratio between children with PAH at baseline assessment and their age-matched controls. Difference between S:D ratio at the 4 predefined time points, changes in S:D ratio over time since diagnosis, and associations between S:D ratio and demographics and clinical and echocardiographic parameters were assessed in univariable linear regression models with a maximum likelihood algorithm for parameter estimation. Regression models were adjusted for repeated measures with an autoregressive covariance structure. Parameter estimate and its SE are reported, where parameter estimate represents the change in S:D ratio for each increase of 1 U in the independent variable (unless otherwise indicated).
Freedom from the composite outcome of clinical worsening, lung transplantation, and death was modeled in Kaplan-Meier nonparametric analyses using S:D ratio at maximum S:D ratio as the dependent variable. The relation of S:D ratio with the specific end points of death or lung transplantation, independent of clinical worsening, was separately analyzed.
Cutpoints for S:D ratio ranges associated with low risk, medium risk, and high risk of clinical outcomes were determined by the inflection point maximizing the segregation of patients by outcomes. A multivariable linear regression model, adjusted for multiple measurements per patient through an autoregressive covariance structure, was used to determine if there were any differences in change in S:D ratio over time between patients with negative clinical outcomes and those without.
To determine the independence of S:D ratio as a predictor of clinical outcomes, individual multivariable models were created including S:D ratio and heart rate, S:D ratio and pulmonary artery pressure, S:D ratio, systolic duration time, and diastolic duration.
To determine intra- and interobserver reliabilities, S:D ratio was measured on 10 consecutive echocardiograms from 10 different patients 2 times by the same observer with an interval of >4 weeks between sittings and by a second observer, respectively. Intra- and interobserver reliabilities were calculated using Bland-Altman statistics for the detection of systematic bias and limits of agreements were defined.
Appropriate mathematical transformations were applied to variables with skewed distribution. A p value <0.05 was considered statistically significant. All statistical analyses were performed using SAS 9.1 (SAS Institute, Cary, North Carolina).
The study was approved by the institutional research ethics board.
Results
Fifty-four patients met inclusion criteria. Of these, 7 (13%) were excluded because of inadequate TR Doppler tracing to measure the duration of systole and diastole, leaving 47 patients for analysis. We reviewed 503 serial echocardiograms from these patients, of which the S:D ratio could be calculated in 382 (76%). Demographic and clinical characteristics of the study population and the 47 age-matched controls are listed in Table 1 . Patients with intracardiac shunts or patent ductus arteriosus who did not undergo surgical repair were not candidates for surgery due to fixed, increased pulmonary vascular resistance. Echocardiographic, catheterization, and 6-minute walk data are listed in Table 2 . Catheter data were available for 22 of 47 patients at the initial evaluation, for 23 of 47 at the second evaluation, for 15 of 47 at the third evaluation, and for 29 of 47 at the latest evaluation.
| Patients (n = 47) | Controls (n = 47) | |
|---|---|---|
| Gender (male) | 15 (32%) | 26 (55%) |
| Age at diagnosis (years) | 5.5 (0–19) | 11 (6–16) |
| Cardiac anatomy | ||
| Structurally normal | 17 (36%) | 47 (100%) |
| Congenital heart disease | 30 (64%) | |
| Type of congenital heart disease | ||
| Atrial septal defect | 4 (14%) | |
| Atrial septal defect and ventricular septal defect | 3 (10%) | |
| Atrioventricular septal defect | 4 (14%) | |
| Atrioventricular septal defect and patent ductus arteriosus | 1 (3%) | |
| Patent ductus arteriosus | 5 (16%) | |
| Patent ductus arteriosus and partial anomalous pulmonary venous connection | 1 (3%) | |
| Ventricular septal defect | 9 (30%) | |
| Ventricular septal defect and patent ductus arteriosus | 3 (10%) | |
| Cardiac repair | 17 (57%) | |
| Clinical outcomes | ||
| Clinical worsening ⁎ | 8 (17%) | |
| Lung transplantation | 4 (9%) | |
| Death | 5 (11%) | |
| Any of these outcomes | 12 (26%) | |
| New York Heart Association class (at initial evaluation) | ||
| I | 16 (35%) | |
| II | 25 (53%) | |
| III | 6 (13%) | |
| IV | 0 (0%) |
⁎ Clinical worsening represents hospitalization associated with pulmonary arterial hypertension, sometimes associated with syncope.
| Patients | Controls | p Value | |||
|---|---|---|---|---|---|
| Echocardiography | |||||
| Age at echocardiography (years) | 152 | 10.5 (5.3–15.7) | 47 | 10.9 (5.9–15.9) | 0.11 |
| Body surface area (m 2 ) | 146 | 1.07 (0.64–1.50) | 46 | 1.23 (0.81–1.65) | 0.49 |
| Right ventricular systolic pressure (mm Hg) | 138 | 86 (56–116) | 44 | 24 (20–28) | <0.001 |
| Blood pressure (mm Hg) | 147 | 97 (83–111) | 39 | 101 (89–113) | 0.25 |
| Right ventricular systolic pressure/blood pressure | 136 | 0.91 (0.56–1.26) | 34 | 0.24 (0.2–0.28) | <0.001 |
| Heart rate (beats/min) | 109 | 82 (63–101) | 45 | 78 (54–102) | 0.49 |
| Systole (ms) | 143 | 405 (344–466) | 47 | 338 (213–405) | <0.001 |
| Diastole (ms) | 143 | 344 (199–489) | 47 | 486 (213–795) | <0.001 |
| Systolic to diastolic duration ratio | 143 | 1.38 (0.77–1.99) | 47 | 0.72 (0.56–0.88) | <0.001 |
| Right ventricular fractional area of change from apical view (%) | 86 | 0.32 (0.17–0.47) | 34 | 0.44 (0.35–0.53) | 0.004 |
| Right ventricular end-diastolic dimension (mm) | 139 | 26 (16–36) | 43 | 18 (13–23) | <0.001 |
| Right ventricular end-diastolic dimension Z-score | 139 | 3.2 (−2.4 to +9.0) | |||
| Right ventricular function | 147 | 47 | |||
| Normal (%) | 111 | 76 | 47 | 100 | <0.001 |
| Mildly decreased (%) | 17 | 11 | |||
| Moderately decreased (%) | 12 | 8 | |||
| Severely decreased (%) | 7 | 5 | |||
| Mean pulmonary artery pressure (mm Hg) | 78 | 51 (30–72) | 33 | 14 (11–17) | <0.001 |
| 6-Minute walk test | |||||
| Distance (m) | 113 | 394 (283–505) | |||
| Oxymetry before walk (%) | 110 | 95 (90–100) | |||
| Oxymetry at peak exercise (%) | 111 | 85 (73–97) | |||
| Oxymetry at recovery (%) | 110 | 95 (90–100) | |||
| Cardiac catheterization | |||||
| Systolic right ventricular pressure (mm Hg) | 72 | 74 (50–98) | |||
| Diastolic right ventricular pressure (mm Hg) | 69 | 8 (4–12) | |||
| Systolic pulmonary artery pressure (mm Hg) | 84 | 73 (48–98) | |||
| Diastolic pulmonary artery pressure (mm Hg) | 83 | 34 (19–49) | |||
| Mean pulmonary artery pressure (mm Hg) | 84 | 52 (34–70) | |||
| Indexed pulmonary blood flow (L/min/m 2 ) | 68 | 3.18 (1.81–4.55) | |||
| Indexed systemic blood flow pulmonary vascular resistance (L/min/m 2 ) | 71 | 2.84 (2.07–3.61) | |||
| Indexed pulmonary blood flow/indexed systemic blood flow pulmonary vascular resistance | 68 | 1.19 (0.58–1.8) | |||
| Pulmonary vascular resistance indexed for body surface area baseline (WU × m 2 ) | 79 | 15.6 (6–25) | |||
| Cardiac magnetic resonance imaging | |||||
| Right ventricular ejection fraction (%) | 7 | 47 (31–63) | |||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


