In light of the low cost, the widespread availability of the electrocardiogram, and the increasing economic burden of the health-related problems, we aimed to analyze the prognostic value of automatic frontal QRS-T angle to predict mortality in patients with left ventricular (LV) systolic dysfunction after acute myocardial infarction (AMI). About 467 consecutive patients discharged with diagnosis of AMI and with LV ejection fraction ≤40% were followed during 3.9 years (2.1 to 5.9). From them, 217 patients (47.5%) died. The frontal QRS-T angle was higher in patients who died (116.6 ± 52.8 vs 77.9 ± 55.1, respectively, p <0.001). The QRS-T angle value of 90° was the most accurate to predict all-cause cardiac death. After multivariate analysis, frontal QRS-T angle remained as an excellent predictor of all-cause and cardiac deaths, increasing the mortality 6% per each 10°. For the global mortality, the hazard ratio for a QRS-T angle >90° was 2.180 (1.558 to 3.050), and for the combined end point of cardiac death and appropriate implantable cardioverter defribrillator therapy, it was 2.385 (1.570 to 3.623). This independent predictive value was maintained even after adjusting by bundle brunch block, ST-elevation AMI, and its localization. In conclusion, a wide automatic frontal QRS-T angle (>90°) is a good discriminator of long-term mortality in patients with LV systolic dysfunction after an AMI. The ability to easily measure it from a standard 12-lead electrocardiogram together with its prognostic value makes the frontal QRS-T angle an attractive tool to help clinicians to improve risk stratification of those patients.
Because the left ventricular ejection fraction (LVEF) is neither highly specific nor highly sensitive as a risk factor for follow-up death, considerable interest exists in identifying novel risk factors that may be more useful than, or adjunctive to, those currently employed. Data from rest electrocardiography may also be used to predict mortality. The determination of the QRS-T angle, although based on a concept already described at the beginning of electrocardiography, has become to be regarded as a useful parameter in clinical practice in the last decade. It has been proposed that a wide angle is a marker of heterogeneity of ventricular repolarization and has been linked to cardiac mortality in the general population. Taking this into consideration, we performed an analysis to investigate the long-term predictive value of the frontal QRS-T to predict cardiac and not cardiac death.
Methods
This was a retrospective study including all patients consecutively discharged from our hospital with diagnosis of acute myocardial infarction (AMI) from 1/2004 to 10/2010. We used the joint consensus document of the European Society of Cardiology and the American College of Cardiology for the standard diagnostic of AMI. From 4,371 patients surviving to in-hospital phase, 494 patients were identified as having LV dysfunction, defined arbitrarily as LVEF <40% at discharge on 2-dimensional echocardiography. Patients with pacemaker (n = 23) were excluded to analyze the intrinsic QRS-T angle. Thus, the final cohort was composed of 471 patients ( Figure 1 ). The study complies with the Declaration of Helsinki and was approved by the Clinical Research Ethics Committee of our hospital.

Demographic, clinical, and angiographic data, as well as data on management and follow-up, were prospectively collected and recorded in an electronic database. Baseline clinical variables were used to evaluate the prognostic value for predicting all-cause mortality.
For each patient, we took the electrocardiogram (ECG) at admission, similar to the methodology used in the Evaluation of Methods and Management of Acute Coronary Events study. We analyzed the computerized values of QRS and T axes. The absolute difference between the frontal QRS and frontal T-wave axes was calculated as T-wave axis − QRS axis and if >180° was subtracted from 360° to give a continuous variable ranging from 0° to 180°.
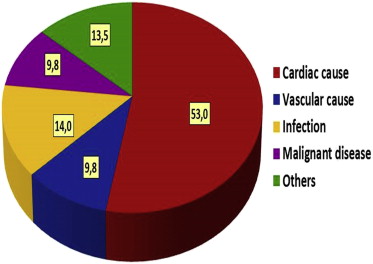
We defined the primary end point as all-cause mortality. Underlying and contributing causes of death were recorded. We used national death certification data and hospital chart or physician’s records to identify all deaths. Cause of death for each patient was classified by 2 clinicians and supported on International Classification of Diseases, 10th revision, codes. We categorized the etiology of death into 5 groups ( Figure 2 ): cardiac (acute coronary syndrome, heart failure, valvular heart disease, and cardiac arrhythmia), vascular (cerebrovascular disease and peripheral artery disease), infection-related death, malignant disease, and other causes.
During follow-up, the occurrence of appropriate implantable cardioverter defibrillator (ICD) therapy was also noted. In those patients, an electrophysiologist determined whether or not the ICD therapy was appropriate. All therapies, either antitachycardia pacing or shock, were classified as appropriate when they occurred in response to life-threatening arrhythmias (ventricular tachycardia or ventricular fibrillation).
The statistical analyses were performed with SPSS 20.0. The categorical or dichotomous variables are expressed as absolute values and percentages and were compared with the Pearson’s chi-square test. The continuous variables are described as mean ± SD or as median and interquartile range. Student t or Mann-Whitney U test was used for the comparisons of continuous variables between 2 groups of patients, as appropriate.
A survival analysis was performed for each end point: (1) follow-up all-cause death and (2) a composite end point of cardiac death and first appropriate device therapy, whichever occurred first. The cutoff point of 90° for the frontal QRS-T angle was defined according to a receiver-operating characteristic curve (optimal QRS-angle to predict mortality). Cumulative event rates of end points were analyzed by the method of Kaplan-Meier (log-rank test). A Cox proportional hazards model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) and assess the performance of the QRS-T angle in a multivariable model. In this model, we included those variables that resulted significant predictors of mortality in the univariable model. A p value <0.05 was considered statistically significant.
Results
Complete follow-up data were available for 99.1% (n = 467) of the 471 patients over a median follow-up time of 3.9 years (interquartile range 2.1 to 5.9). The mean age was 70.0 ± 12.5, with 24.6% women and 35.3% diabetics. The rate of ST-segment elevation myocardial infarction (STEMI) was 47.3%, and the mean of LVEF was 34.4 ± 5.8, with 51.6% of patients in Killip class ≥II. From those 457 patients, 217 (47.5%) died during follow-up. Baseline characteristics according to survivors and nonsurvivors are listed in Table 1 .
| Variable | Overall Population | Exitus (n = 217) | No-Exitus (n = 250) | p Value |
|---|---|---|---|---|
| Age (yrs) | 70.0 ± 12.5 | 76.3 ± 10.4 | 65.8 ± 12.2 | <0.001 |
| Female sex | 115 (24.6) | 55 (25.3) | 60 (24.0) | 0.736 |
| Diabetes mellitus | 165 (35.3) | 93 (42.9) | 72 (28.8) | 0.002 |
| Previous coronary artery disease | 147 (31.5) | 91 (41.9) | 56 (22.4) | <0.001 |
| Previous heart failure | 75 (16.1) | 54 (24.9) | 21 (8.4) | <0.001 |
| Peripheral artery disease | 77 (16.5) | 50 (23.0) | 27 (10.8) | <0.001 |
| Chronic obstructive pulmonary disease | 65 (13.9) | 47 (21.7) | 18 (7.2) | <0.001 |
| Previous malignant disease | 40 (8.6) | 31 (14.3) | 9 (3.6) | <0.001 |
| STEMI | 221 (47.3) | 88 (40.6) | 133 (53.2) | 0.006 |
| Killip ≥2 | 241 (51.6) | 129 (59.4) | 112 (44.8) | 0.002 |
| LVEF | 34.4 ± 5.8 | 33.7 ± 5.9 | 34.9 ± 5.8 | 0.021 |
| Troponine I peak (ng/dl) | 60.8 ± 97.8 | 42.8 ± 75.5 | 76.8 ± 111.6 | <0.001 |
| Atrial fibrillation | 81 (17.3) | 47 (21.7) | 34 (13.6) | 0.022 |
| Left bundle branch block | 62 (13.4) | 38 (17.6) | 24 (9.7) | 0.012 |
| Right bundle branch block | 46 (9.9) | 27 (12.5) | 19 (7.7) | 0.082 |
| QRS-T angle | 95.9 ± 57.3 | 116.6 ± 52.8 | 77.9 ± 55.1 | <0.001 |
| Anterior STEMI | 152 (32.5) | 56 (25.8) | 96 (38.4) | 0.004 |
| Multivessel disease | 217 (46.5) | 102 (47.0) | 115 (46.0) | 0.828 |
| Main left coronary artery | 38 (8.1) | 20 (9.2) | 18 (7.2) | 0.427 |
| Percutaneous coronary intervention | 297 (63.6) | 111 (51.2) | 186 (74.4) | <0.001 |
| Coronary artery bypass grafting | 27 (5.8) | 7 (3.2) | 20 (8.0) | 0.027 |
| MDRD-4 (ml/min/1.73 m 2 ) | 65.9 ± 25.3 | 71.8 ± 23.3 | 59.2 ± 25.9 | <0.001 |
| Aspirin | 406 (86.9) | 174 (80.2) | 232 (92.8) | <0.001 |
| Clopidogrel | 352 (74.9) | 152 (70.0) | 200 (79.2) | 0.023 |
| β blocker | 308 (66.0) | 119 (54.8) | 189 (75.6) | <0.001 |
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers | 350 (74.9) | 149 (68.7) | 201 (80.4) | 0.004 |
| Antialdosteronic | 120 (25.7) | 51 (23.5) | 69 (27.6) | 0.312 |
| Statin | 378 (80.9) | 161 (74.2) | 217 (86.8) | <0.001 |
The baseline frontal QRS-T angle was 95.9 ± 57.3°. It was higher in patients who died (116.6 ± 52.8 vs 77.9 ± 55.1, respectively, p <0.001). In receiver-operating characteristic curves ( Figure 3 ), the most accurate value of frontal QRS-T angle to predict all-cause cardiac death was 90°, with an area under the curve of 0.690 ± 0.025 (sensitivity and specificity of 73.3% and 62.2%, respectively). In 251 patients (53.7%), the frontal QRS-T angle was higher than 90°, with a higher rate of mortality in comparison with patients with QRS-T angle ≤90° (73.3% vs 26.7%, respectively, p <0.001; Figure 4 ). As listed in Table 2 , patients with a wide frontal QRS-T angle (>90°) were more likely to be older, diabetic, with previous coronary artery disease, previous heart failure, and peripheral artery disease, to present with higher rate of non–ST-segment elevation myocardial infarction, to have a lower LVEF, and to have a more percentage of left bundle branch block. After adjusting by those variables that were associated with follow-up death in univariate analysis ( Table 3 ), automatic frontal QRS-T angle remained as an excellent predictor of all-cause and cardiac deaths (HR 1.006, 95% CI 1.003 to 1.009, p <0.001), increasing the mortality 6% per each 10° ( Figure 5 ). A frontal QRS-T angle >90° increases the risk of all-cause mortality by twofold (HR 2.180, 95% CI 1.558 to 3.050, p <0.001), after adjusting by the previously described variables.
| Variable | QRS-T Angle ≤90° (n = 216) | QRS-T Angle >90° (n = 251) | p Value |
|---|---|---|---|
| Age (yrs) | 67.9 ± 13.0 | 73.1 ± 11.6 | <0.001 |
| Female sex | 56 (25.9) | 59 (23.5) | 0.545 |
| Diabetes mellitus | 61 (28.2) | 104 (41.4) | 0.003 |
| Previous coronary artery disease | 46 (21.3) | 101 (40.2) | <0.001 |
| Previous heart failure | 19 (8.8) | 56 (22.3) | <0.001 |
| Peripheral artery disease | 25 (11.6) | 52 (20.7) | 0.008 |
| Chronic obstructive pulmonary disease | 30 (13.9) | 35 (13.9) | 0.986 |
| Previous malignant disease | 13 (6.0) | 27 (10.8) | 0.068 |
| STEMI | 143 (66.2) | 78 (31.1) | <0.001 |
| Killip ≥2 | 99 (45.8) | 142 (56.6) | 0.021 |
| LVEF | 35.7 ± 4.8 | 33.2 ± 6.4 | <0.001 |
| Troponine I peak (ng/dl) | 87.7 ± 115.8 | 37.7 ± 71.6 | <0.001 |
| Atrial fibrillation | 28 (13.0) | 53 (21.1) | 0.020 |
| Left bundle branch block | 5 (2.3) | 57 (22.9) | <0.001 |
| Right bundle branch block | 15 (7.0) | 31 (12.4) | 0.049 |
| Anterior STEMI | 95 (44.0) | 57 (22.7) | <0.001 |
| Multivessel disease | 101 (46.8) | 116 (46.2) | 0.906 |
| Main left coronary artery | 11 (5.1) | 27 (10.8) | 0.026 |
| Percutaneous coronary intervention | 166 (76.9) | 131 (52.2) | <0.001 |
| Coronary artery bypass grafting | 12 (5.6) | 15 (6.0) | 0.846 |
| MDRD-4 (ml/min/1.73 m 2 ) | 69.6 ± 23.8 | 62.8 ± 26.2 | 0.003 |
| Aspirin | 200 (92.6) | 206 (82.1) | 0.001 |
| Clopidogrel | 174 (79.6) | 178 (70.9) | 0.030 |
| β blocker | 147 (68.1) | 161 (64.1) | 0.374 |
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers | 172 (79.6) | 178 (70.9) | 0.030 |
| Antialdosteronic | 53 (24.5) | 67 (26.7) | 0.595 |
| Statin | 190 (88.0) | 188 (74.9) | <0.001 |
| Variable | HR | 95% CI | p Value |
|---|---|---|---|
| Age (yrs) | 1.053 | 1.037–1.070 | <0.001 |
| Diabetes mellitus | 1.237 | 0.931–1.645 | 0.143 |
| Previous coronary artery disease | 1.363 | 1.013–1.835 | 0.041 |
| Previous heart failure | 0.983 | 0.676–1.429 | 0.927 |
| Peripheral artery disease | 1.104 | 0.762–1.599 | 0.600 |
| Chronic obstructive pulmonary disease | 1.399 | 0.984–1.988 | 0.061 |
| Previous malignant disease | 1.401 | 0.938–2.092 | 0.099 |
| STEMI | 1.300 | 0.926–1.825 | 0.130 |
| Killip ≥2 | 1.039 | 0.761–1.419 | 0.809 |
| LVEF (%) | 0.984 | 0.961–1.007 | 0.168 |
| Troponine I peak (ng/dl) | 0.999 | 0.997–1.001 | 0.221 |
| Atrial fibrillation | 0.874 | 0.612–1.249 | 0.461 |
| Left bundle branch block | 1.023 | 0.677–1.547 | 0.914 |
| QRS-T angle (per each degree) | 1.006 | 1.003–1.009 | <0.001 |
| Anterior STEMI | 0.880 | 0.554–1.397 | 0.587 |
| Percutaneous coronary intervention | 0.706 | 0.501–0.994 | 0.046 |
| Coronary artery bypass grafting | 0.678 | 0.305–1.511 | 0.342 |
| MDRD-4 (ml/min/1.73 m 2 ) | 0.992 | 0.986–0.998 | 0.011 |
| Aspirin | 0.697 | 0.466–1.040 | 0.077 |
| Clopidogrel | 1.467 | 0.998–2.155 | 0.051 |
| β blocker | 0.701 | 0.522–0.942 | 0.019 |
| Angiotensin-converting enzyme inhibitors/angiotensin receptor blockers | 0.930 | 0.658–1.315 | 0.682 |
| Antialdosteronic | 1.107 | 0.784–1.564 | 0.564 |
| Statin | 0.611 | 0.432–0.864 | 0.005 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


