In this study, we tested the hypothesis that impaired midwall shortening predicts cardiovascular (CV) mortality in patients with type 2 diabetes mellitus (DM). In patients with DM without overt cardiac disease, systolic left ventricular (LV) function analyzed by midwall shortening may be impaired although LV ejection fraction is preserved. Impaired midwall shortening is an early independent prognosticator of adverse clinical outcome in patients with arterial hypertension. We analyzed the echocardiographic data from 360 outpatients with DM collected during the years 1990 to 2007. Patients had no history or symptoms attributable to cardiac disease. Stress-corrected midwall shortening (sc-MS) was taken as index of systolic LV function and considered impaired if <89%. The study outcome was CV mortality. At baseline, impaired sc-MS was detected in 140 patients (39%). During a mean follow-up period of 11 years, 54 patients (15%) died, 31 (8.6%) of them from CV causes. CV deaths occurred in 21 of 140 patients (15%) with impaired sc-MS and in 10 of 220 patients (4.5%) with normal sc-MS (p = 0.006). Multivariate Cox regression analysis revealed that impaired sc-MS (hazard ratio 1.03, 95% confidence interval 1.01 to 1.08, p = 0.039), together with lower estimated glomerular filtration rate (hazard ratio 0.96, 95% confidence interval 0.93 to 0.99, p = 0.004), was independently associated with CV mortality even after adjustment for age, diabetes duration, hemoglobin A 1c , left atrial diameter, and heart valve calcium. In conclusion, subclinical systolic LV dysfunction as measured by sc-MS occurs frequently in patients with DM without overt cardiac disease and independently predicts long-term CV mortality in such patients together with lower estimated glomerular filtration rate.
A remarkable proportion of patients with type 2 diabetes mellitus (DM) without overt cardiac disease have asymptomatic left ventricular (LV) systolic dysfunction although conventional echocardiographic indexes of chamber function such as LV ejection fraction are normal. This condition is revealed by the assessment of stress-corrected midwall shortening (sc-MS), which identifies early systolic impairment of circumferential LV myocardial fibers. The impairment of sc-MS is an early and reliable indicator of the transition phase between normal cardiac function and clinically manifest congestive heart failure (HF) as well as a potent predictor of adverse cardiovascular (CV) outcomes both in patients with hypertension and in those with chronic HF but preserved LV ejection fraction. To our knowledge, nobody has investigated the impact of sc-MS on prognosis in people with DM. Accordingly, this observational study has been conceived to analyze the prevalence and clinical characteristics of patients with DM with impaired versus normal sc-MS and to assess whether impaired sc-MS predicts CV mortality in the absence of baseline overt CV disease.
Methods
The present research was conducted within the frame of the Verona Diabetes Study, an observational longitudinal investigation on chronic complications in outpatients with DM who regularly attend the diabetes clinic of the Verona University Hospital. For the present substudy, we selected patients free of overt CV disease and other diseases that could jeopardize the follow-up. Accordingly, we have examined the electronic records of all Caucasian outpatients with established DM who had been referred for a standard transthoracic echocardiography because of clinical reasons at our institution during the years 1990 to 2007. Among the 937 initially eligible subjects, we excluded 508 patients who had a history of myocardial infarction, myocardial revascularization or coronary artery disease, dilated cardiomyopathy or HF, primary hypertrophic or restrictive cardiomyopathy, or chest pain with exercise test showing inducible myocardial ischemia. Another 35 patients with clinically significant valve disease or with aortic and/or mitral valve prostheses, and 34 patients who had asymptomatic abnormalities of LV wall motion were also excluded. As a result of this selection, 360 outpatients with DM met the predefined inclusion criteria and were included in the final analysis. All subjects were in sinus rhythm, free of symptoms and clinical signs of CV disease or diagnosis of cancer at enrollment. Clinical history and physical and routine laboratory examinations were available in all participants. Glomerular filtration rate (GFR) was estimated from the 4-variable Modification of Diet in Renal Disease study equation. Urinary albumin excretion rate was measured from a 24-hour urine sample using an immunonephelometric method and the presence of abnormal albuminuria was defined as albumin excretion rate >30 mg/day. Chronic kidney disease was defined as the presence of estimated GFR <60 ml/min/1.73 m 2 . The presence of retinopathy was diagnosed by fundoscopy by a single ophthalmologist. Subjects were considered to have arterial hypertension if their blood pressure was ≥140/90 mm Hg or if they were taking any antihypertensive drugs. Obesity was diagnosed if patients had a body mass index of ≥30 kg/m 2 . The study protocol was approved by local ethics committee. The informed consent requirement for this study was exempted by the ethics committee because researchers only accessed retrospectively a deidentified database for analysis purposes. The study protocol conforms to the ethical guidelines of the Declaration of Helsinki as revised in 2000.
All echocardiographic examinations were performed following a standardized protocol by experienced cardiologists. LV chamber dimensions and wall thicknesses were measured by the American Society of Echocardiography guidelines and LV mass was calculated using a necropsy-validated formula. LV mass was normalized for height to the 2.7 power and LV hypertrophy was defined as LV mass ≥49.2 g/m 2.7 for men and ≥46.7 g/m 2.7 for women. Relative wall thickness was calculated as 2 × end-diastolic ratio posterior wall thickness/LV diameter and indicated concentric LV geometry if ≥0.43 (the 97.5 percentile in a normal population).
LV ejection fraction was measured in each patient from LV 2-dimensional area changes in systole and diastole (area-length method) and defined as reduced if <50%. Systolic LV function was also assessed during the same examination by measuring the systolic shortening of the LV minor axis at the midwall level (midwall shortening). Midwall shortening was calculated taking into account the epicardial migration of the midwall during systole caused by the architectural organization of myocardial fibers, as previously described. Midwall end-systolic circumferential stress was calculated and related to midwall shortening to assess afterload-independent LV systolic function, as previously reported. Sc-MS <89% (corresponding to the ninetieth percentile distribution of sc-MS in a healthy population with similar age and prevalence of female gender, previously analyzed in our center) was considered indicative of subclinical systolic LV dysfunction.
Left atrial diameter was measured from M-mode tracing from the parasternal longitudinal view. Mitral annular calcium was defined by increased echo density located at the atrioventricular junction and posterior mitral leaflet on the parasternal long- or short-axis or apical four-chamber view. Aortic valve sclerosis was defined as focal or diffuse calcium and thickening of aortic leaflets with or without restriction of leaflet motion.
Vital status and cause of death on September 30, 2007 (which could be clearly identified in 100% of subjects) were ascertained for all participants through examination of the electronic databases of the Public Health Unit of the Veneto Region. Death certificates were coded by trained nosologists using the International Classification of Diseases, ninth revision. Deaths were attributed to CV causes when International Classification of Diseases, ninth revision, codes were 390 to 459. A selected sample of death certificates was reviewed manually to validate the process. Deaths for CV causes were the primary outcome of the study.
Data are reported as means ± SD (medians and interquartile ranges for variables deviating from normality) or percentages. The study population was stratified by status of sc-MS at baseline. The cut-off value for impaired sc-MS was a priori identified as <89%, as previously reported. Between-group comparisons of categorical and continuous variables were performed by chi-square tests and analysis of variance with comparison between groups by Scheffé test and Tukey honestly significant difference test for unequal samples, as appropriate. Multivariate logistic regression analysis was performed to identify the factors independently related to impaired sc-MS at baseline. The following variables were included as covariates in the adjusted regression model: age, gender, diastolic blood pressure, high-density lipoprotein (HDL) cholesterol, glycated hemoglobin, concentric LV geometry, LV hypertrophy, LV end-diastolic diameter, and presence of aortic and/or mitral valve calcium. Kaplan-Meier survival curves were performed and the differences were tested for significance by the log-rank test. Log cumulative hazard functions were also computed by univariate and multivariate Cox proportional hazards analyses to identify the predictors of CV mortality and probabilities of event-free survival. In a preliminary analysis, considering the dependence of sc-MS on LV concentric geometry and mass, we tested sc-MS together with relative wall thickness (used as index of concentric remodeling) and LV hypertrophy. In the final model, along with sc-MS that was included as a categorical variable (<89% vs ≥89%), other variables included in multivariate Cox regression models were selected as possible confounding factors on the basis of their significance in univariate analyses: age, diabetes duration, glycated hemoglobin, estimated GFR, and aortic and/or mitral valve calcium. In another Cox regression model, LV ejection fraction was also forcedly added to the variables listed previously. All analyses were performed using statistical package SPSS 19.0 (SPSS Inc., Chicago, Illinois) and statistical significance was identified by 2-tailed p <0.05.
Results
The baseline clinical and echocardiographic characteristics of the participants stratified by sc-MS status are listed in Table 1 . Impaired sc-MS was detected in 140 patients (38.9% of total) who had significantly lower diastolic blood pressure and HDL cholesterol and tended to have higher low-density lipoprotein cholesterol than those with normal sc-MS. As also listed in Table 1 , compared with patients with normal sc-MS, those with impaired sc-MS had significantly smaller LV diameters and higher LV relative wall thickness and mass and were more likely to have mitral and/or aortic valve calcium as well as LV hypertrophy and concentric LV geometry.
| Variable | Impaired Sc-MS (<89%, n = 140) | Normal Sc-MS (≥89%, n = 220) | p Value |
|---|---|---|---|
| Age (yrs) | 68 ± 10 | 67 ± 10 | 0.75 |
| Women | 35 | 45 | 0.07 |
| BMI (kg/m 2 ) | 29 ± 6 | 29 ± 5 | 0.50 |
| Obesity (BMI ≥30 kg/m 2 ) | 32 | 36 | 0.48 |
| Hypertension | 92 | 90 | 0.21 |
| Current smoker | 22 | 16 | 0.13 |
| Diabetes duration (yrs) | 15 (9–20) | 15 (11–23) | 0.39 |
| Diabetic retinopathy | 34 | 25 | 0.15 |
| Systolic blood pressure (mm Hg) | 139 ± 20 | 141 ± 20 | 0.32 |
| Diastolic blood pressure (mm Hg) | 79 ± 10 | 81 ± 11 | 0.03 |
| Fasting glucose (mmol/L) | 8.5 (7.0–10.1) | 8.7 (7.2–10.5) | 0.30 |
| Glycated hemoglobin, % (mmol/mol) | 7.6 ± 1.4 (60) | 7.4 ± 1.5 (58) | 0.25 |
| HDL cholesterol | <0.001 | ||
| mmol/L | 1.18 (1.01–1.45) | 1.34 (1.17–1.60) | |
| mg/dl | 46 (39–56) | 52 (45–62) | |
| LDL cholesterol | 0.06 | ||
| mmol/L | 4.79 (4.32–5.33) | 5.01 (4.31–5.74) | |
| mg/dl | 185 (167–206) | 194 (167–222) | |
| Triglycerides | 0.62 | ||
| mmol/L | 1.56 (1.15–2.04) | 1.44 (0.99–2.09) | |
| mg/dl | 138 (102–181) | 128 (88–185) | |
| Estimated GFR (ml/min/1.73 m 2 ) | 65 ± 19 | 66 ± 18 | 0.66 |
| Albuminuria (mg/day) | 64 ± 125 | 73 ± 160 | 0.22 |
| Albuminuria | 43 | 35 | 0.37 |
| Antiplatelet agents | 60 | 54 | 0.86 |
| Statin users | 54 | 53 | 0.25 |
| Echocardiographic parameters | |||
| LV end-diastolic diameter (cm/h) | 2.9 ± 0.4 | 3.1 ± 0.3 | <0.001 |
| LV end-systolic diameter (cm/h) | 1.9 ± 0.5 | 1.8 ± 0.3 | 0.05 |
| Relative wall thickness | 0.46 ± 0.08 | 0.36 ± 0.06 | <0.001 |
| Concentric LV geometry ∗ | 54 | 16 | <0.001 |
| LV mass index (g/m 2.7 ) | 58 ± 16 | 51 ± 12 | <0.001 |
| LV hypertrophy | 74 | 59 | 0.007 |
| LV ejection fraction (%) | 58 ± 10 | 66 ± 7 | <0.001 |
| Midwall shortening (%) | 14.2 ± 2.0 | 19.0 ± 2.0 | <0.001 |
| CESS (kdynes/cm 2 ) | 128 ± 46 | 143 ± 38 | 0.003 |
| sc-MS (%) | 76 ± 15 | 103 ± 19 | <0.001 |
| Left atrial diameter (mm/h) | 24.0 ± 3.5 | 24.2 ± 3.5 | 0.53 |
| Mitral valve calcium | 23 | 12 | 0.02 |
| Aortic valve calcium | 41 | 26 | 0.03 |
| Aortic and mitral calcium | 16 | 8 | <0.001 |
A multivariate logistic regression analysis was performed to identify the variables that were independently associated with impaired sc-MS at baseline. The presence of concentric LV geometry (adjusted odds ratio 7.09, 95% confidence interval [CI] 3.9 to 12.9, p <0.001), LV hypertrophy (odds ratio 2.69, 95% CI 1.4 to 5.0, p = 0.002), and lower serum HDL cholesterol level (odds ratio 0.37, 95% CI 0.15 to 0.91, p = 0.03) were independently associated with impaired sc-MS at baseline.
During a mean follow-up period of 11 ± 5 years, 54 patients (15% of total) died, 31 of them from CV causes. The 23 non-CV deaths were incident cancer in 11, lung diseases in 4, cachexia in 4, kidney failure in 2, and liver failure in 2 patients. Reasons of the 31 CV deaths were acute myocardial infarction in 11 patients, HF in 9, sudden death in 8, ischemic stroke in 2, and severe aortic valve disease in 1 patient.
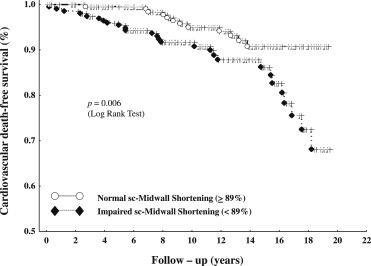
Remarkably, 21 of the 31 CV deaths occurred in the 140 patients with impaired sc-MS (15%) and 10 in the 220 patients with normal sc-MS (4.5%, p = 0.006 for the difference between the groups). This difference was substantially due to deaths from HF, which occurred in 8 patients with impaired sc-MS (5.7%) and only in 1 patient with normal sc-MS at baseline (0.4%, p <0.001), whereas the frequency of the other causes of CV death differed less between the 2 study groups. Figure 1 shows the CV death-free survival curves of patients with baseline impaired or normal sc-MS (p = 0.006 by log-rank test).
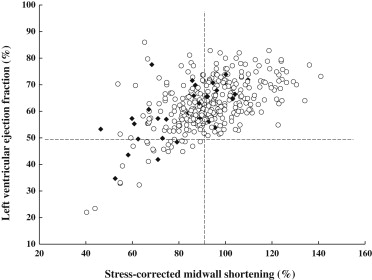
Considering LV ejection fraction as index of systolic function, it was found normal at baseline evaluation in 334 patients (93%) and reduced (<50%) in 26 participants (7%). CV death occurred in 4 of these 26 subjects (15.4%) and in 27 of 334 patients with normal LV ejection fraction (8.1%, p = 0.21). Figure 2 shows the distribution of patients who died of CV causes during the follow-up and those who did not according to the values of LV ejection fraction and sc-MS. Risk for CV death was 1.9-fold higher in patients with reduced than in those with normal LV ejection fraction and 3.3-fold higher in patients with reduced than in those with normal sc-MS.