Left ventricular (LV) remodeling represents an important determinant in the progression to heart failure in patients after myocardial infarction. The aim of the present study was to evaluate in patients with first ST-segment elevation acute myocardial infarction who were successfully and completely reperfused whether the control of cholesterol is predictive of LV remodeling. A total of 109 patients referred to a coronary care unit for first ST-segment elevation myocardial infarction were analyzed. According to the change in indexed LV end-diastolic volume detected at follow-up visits, patients were divided into nonremodeling (n = 79) and remodeling (n = 30) groups. At coronary care unit admission, the prevalence of cardiovascular risk factors was similar in the 2 groups. Low-density lipoprotein (LDL) cholesterol values were used as criteria for cholesterol control. At follow-up visits, the prevalence of patients with target levels of plasma LDL cholesterol was lower in the remodeling compared to the nonremodeling group (67% and 91%, respectively, p <0.01). After adjusting for age, gender, baseline LV ejection fraction, baseline indexed LV end-diastolic volume, hypertension, diabetes, obesity, smoking status, time from acute event, drugs (β blockers, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, calcium channel blockers, and statins), wall motion score index, and troponin levels, logistic regression analysis showed that patients with nontarget LDL cholesterol values at follow-up were significantly more likely to show cardiac remodeling (odds ratio 22.3, 95% confidence interval 2.91 to 171.9, p = 0.003). In conclusion, the present study shows that unsatisfactory control of LDL cholesterol independently predicts LV remodeling in patients with first ST-segment elevation myocardial infarction.
The present study was performed to evaluate whether the control of cholesterol is predictive of left ventricular (LV) remodeling in patients with first ST-segment elevation acute myocardial infarction (STEMI) who were successfully and completely reperfused.
Methods
We retrospectively screened out all consecutively patients referred from May 2005 to May 2009 for acute STEMI to the coronary care unit (CCU) of the Department of Clinical Medicine, Cardiovascular and Immunologic Sciences at the University of Naples “Federico II.” In our CCU, medical history, demographic and anthropomorphic characteristics, vital signs, hemodynamic and laboratory parameters, and the results of other diagnostic procedures collected during the hospitalization and at follow-up visits are routinely stored in a dedicated database. All data were checked for missing or contradictory entries and values out of the normal range.
The research protocol was approved by the ethics committee of our institution. All patients gave written consent for inclusion into the study.
All patients who met the following inclusion criteria were asked to return to the outpatient clinic of our Department to perform exercise testing and laboratory tests and to undergo Doppler echocardiography ≥6 months after discharge: (1) confirmed first STEMI, (2) successful percutaneous coronary intervention (PCI) <6 hours after the onset of symptoms, (3) successful rescue PCI, (4) successful PCI <24 hours from the onset of symptoms in case of effective thrombolysis, and (5) adequate echocardiographic image quality recorded within the first 24 hours of admission to the CCU. Exclusion criteria were (1) previous myocardial infarction or revascularization, (2) valvular heart disease, (3) evidence of heart failure, (4) need for surgical revascularization, and (6) evidence of exercise-induced ischemia at follow-up visit.
Repeated measurements of biomarkers of cardiac cytolysis were performed within the first 96 hours of admission to the CCU. During hospitalization, serum creatinine and fasting plasma glucose were measured once daily. Serum total cholesterol, high-density lipoprotein cholesterol, triglycerides, and high-sensitivity C-reactive protein were determined the day after admission to the CCU, after a 12-hour overnight fast. Low-density lipoprotein (LDL) cholesterol was calculated using the Friedewald formula.
At the follow-up visit, patients underwent physical examination, exercise stress testing, echocardiography, and determination of serum creatinine, fasting plasma glucose, total cholesterol, LDL cholesterol, high-density lipoprotein cholesterol, and triglycerides.
All patients underwent echocardiography <24 hours after admission to the CCU. Echocardiography was performed using commercially available equipment (Vivid 7; GE Vingmed Ultrasound AS, Horten, Norway) with dedicated 2.5-MHz transducers, with the patient supine in the left lateral position. All echocardiograms were performed in the CCU by experienced ultrasonographers and repeated at the follow-up visits by the same operator. Two experienced investigators who were unaware of patients’ clinical data analyzed echocardiographic images (L.S. and S.D.M.). Intraobserver and interobserver variability values in our laboratory have been previously reported. LV volumes and the LV ejection fraction were calculated from 4- and 2-chamber views using the modified biplane Simpson’s method. To evaluate regional systolic function, the left ventricle was divided according to the 16-segment model recommended by the American Society of Echocardiography. For each segment, wall motion was scored from 1 (normal) to 4 (dyskinetic). Wall motion score index was derived from the sum of segmental scores divided by the number of segments. Dyssynergy was quantified by dividing the number of akinetic and dyskinetic segments by the total number of segments assessed and multiplying by 100. Two-dimensionally guided M-mode LV internal dimension and interventricular septal and posterior wall thicknesses were measured at end-diastole according to the recommendations of the American Society of Echocardiography on 3 cardiac cycles at or just below the tips of the mitral leaflets in parasternal long-axis and short-axis views. LV mass was estimated using the formula developed by Devereux et al. LV mass was divided by body surface area to calculate LV mass index. We measured the following variables from mitral inflow Doppler tracings obtained with the sample volume at the mitral leaflet tips: peak velocity of early transmitral inflow wave (E), peak flow velocity of late transmitral inflow wave (A), E/A ratio, and deceleration time of early filling.
Diagnosis of STEMI was established according criteria elsewhere reported.
Successful PCI was defined as Thrombolysis In Myocardial Infarction (TIMI) grade 3 flow and residual stenosis <30% in the infarct-related artery. Thrombolysis was deemed successful if the patient had no pain and the resolution of ST-segment elevation was observed on an electrocardiogram recorded 90 minutes after administration. Patients with unsuccessful thrombolysis underwent rescue PCI immediately. In those with successful thrombolysis, coronary angiography was performed in the following 24 hours.
LV remodeling was defined, according current research as an increase of indexed LV end-diastolic volume (LVEDVi) ≥20%. Target levels of LDL cholesterol in postinfarction patients were considered those recommended by the National Cholesterol Education Program Adult Treatment Panel III (<100 mg/dl).
Type 2 diabetes mellitus was diagnosed if fasting plasma glucose was ≥126 mg/dl, confirmed by repeated measurement at hospital discharge, or if patients were taking insulin or hypoglycemic medications. Subjects were classified as hypertensive when taking antihypertensive drugs or when the average of the 3 measurements was ≥140 mm Hg for systolic blood pressure and/or ≥90 mm Hg for diastolic blood pressure on ≥2 separate clinic visits during hospitalization.
Hypercholesterolemia was defined as a serum cholesterol level >200 mg/dl or the use of lipid-lowering agents. Self-reported history of diabetes, hypercholesterolemia, and hypertension, as well as self-reported medication use, was cross-checked with the records of patients’ general practitioners.
Chronic kidney disease was defined as an estimated glomerular filtration rate ≤89 ml/min/1.73 m 2 . Glomerular filtration rate was calculated using the Modification of Diet in Renal Disease (MDRD) formula. LV hypertrophy was diagnosed if indexed LV mass was >110 and >130 g/m 2 in women and men, respectively. LV restrictive filling pattern was recognized in the presence of an E/A ratio ≥1 associated with deceleration time ≤150 ms. Patients were defined as obese if they had body mass indexes ≥30.0 kg/m 2 .
All patients underwent exercise stress testing on a bicycle ergometer with an initial workload of 25 W and subsequent increments of 25 W every 2 minutes A 12-lead electrocardiogram and blood pressure values were recorded at baseline and every minute thereafter. Criteria for interrupting the test were chest pain, diagnostic ST-segment shift, fatigue, excessive blood pressure increase (systolic blood pressure >240 mm Hg, diastolic blood pressure >140 mm Hg), limiting dyspnea, or achievement of maximal predicted heart rate in the absence of ischemia.
Data are expressed as mean ± SD or as proportions. Comparisons were performed using paired Student’s t tests, chi-square tests, or Fisher’s exact tests as required. Correlations between variables were assessed using Pearson’s correlation coefficient. Changes in LVEDVi were categorized in quartiles; analysis of variance with Bonferroni’s post hoc test was used to compare LDL cholesterol levels in each quartile. To test the hypothesis that nontarget LDL cholesterol values were predictors of LV remodeling, patients were categorized according to LDL cholesterol control (target or nontarget LDL values). Logistic regression analysis was used to identify predictors LV remodeling (categorized as the presence or absence of LV remodeling at the follow-up visit). Multicollinearity among the covariates was tested using the variance inflation factor. All analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina), with significance set at p <0.05.
Results
Among 714 subjects consecutively referred to the CCU for STEMI from May 2005 to May 2009, we analyzed 109 patients. Forty-six patients died in the CCU, and 28 died after hospital discharge. The reasons for exclusion from the analysis were not meeting the inclusion criteria in 432 patients, refusal of follow-up visit in 55, incomplete medical histories in 12, and poor-quality ultrasound evaluations in 32.
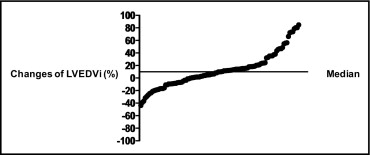
Changes in LVEDVi detected at follow-up had a bimodal distribution, with a median value of 9.98% (range −43.81% to 84.83%; Figure 1 ). Patients were categorized into 2 groups: nonremodeling (n = 79) and remodeling (n = 30).
Demographic, clinical, and biohumoral characteristics of the study population are listed in Table 1 . Coronary artery disease–related characteristics of the study population are listed in Table 2 . In patients treated with PCI, the pain-to-balloon times were 136 ± 183 and 113 ± 149 minutes in the nonremodeling and remodeling groups, respectively (p = 0.63). In those treated with thrombolysis, the pain-to-needle times were 121 ± 71 and 125 ± 91 minutes in the nonremodeling and remodeling groups, respectively (p = 0.95). In patients treated with rescue PCI, the pain-to-reperfusion times were 314 ± 135 and 337 ± 144 minutes in the nonremodeling and remodeling groups, respectively (p = 0.87). Two patients treated with thrombolysis had normal coronary vessels on angiography. The pharmacologic therapy prescribed at hospital discharge was similar in the 2 groups ( Table 2 ). None of the recruited patients underwent exercise-based cardiac rehabilitation programs.
| Characteristic | Overall Population (n = 109) | LV Nonremodeling Group (n = 79) | LV Remodeling Group (n = 30) | p Value |
|---|---|---|---|---|
| Age (years) | 56 ± 10 | 54 ± 10 | 60 ± 10 | 0.02 |
| Men | 91 (83%) | 63 (80%) | 28 (93%) | 0.146 |
| Systolic blood pressure (mm Hg) | 127 ± 21 | 130 ± 22 | 123 ± 19 | 0.194 |
| Diastolic blood pressure (mm Hg) | 79 ± 13 | 80 ± 13 | 76 ± 14 | 0.178 |
| Heart rate (beats/min) | 75 ± 14 | 76 ± 15 | 72 ± 12 | 0.210 |
| Body mass index (kg/m 2 ) | 28 ± 4 | 28 ± 4 | 29 ± 3 | 0.282 |
| Total cholesterol (mg/dl) | 188 ± 40 | 188 ± 38 | 187 ± 44 | 0.870 |
| High-density lipoprotein cholesterol (mg/dl) | 43 ± 11 | 41 ± 11 | 48 ± 12 | 0.004 |
| LDL cholesterol (mg/dl) | 116 ± 39 | 115 ± 34 | 113 ± 44 | 0.716 |
| Triglycerides (mg/dl) | 151 ± 89 | 158 ± 97 | 132 ± 60 | 0.170 |
| Glucose (mg/dl) | 131 ± 58 | 134 ± 62 | 125 ± 46 | 0.507 |
| Creatinine (mg/dl) | 0.9 ± 0.4 | 0.9 ± 0.1 | 1 ± 0.7 | 0.022 |
| Glomerular filtration rate (ml/min/1.73 m 2 ) | 92 ± 21 | 94 ± 20 | 85 ± 21 | 0.032 |
| High-sensitivity C-reactive protein (mg/L) | 13 ± 21 | 11 ± 18 | 17 ± 25 | 0.281 |
| Essential hypertension (blood pressure ≥140/90 mm Hg) | 39 (36%) | 30 (38%) | 9 (30%) | 0.507 |
| Diabetes mellitus (fasting glucose ≥126 mg/dl) | 17 (16%) | 14 (18%) | 3 (10%) | 0.390 |
| Hypercholesterolemia (serum cholesterol >200 mg/dl) | 38 (36%) | 28 (35%) | 10 (33%) | 1 |
| Obesity (body mass index ≥30 kg/m 2 ) | 35 (32%) | 25 (32%) | 10 (33%) | 1 |
| Current smokers | 74 (68%) | 58 (73%) | 16 (53%) | 0.065 |
| LV hypertrophy | 61 (56%) | 44 (56%) | 17 (57%) | 1 |
| Chronic kidney disease | 4 (3.7%) | 3 (4%) | 1 (3.4) | 1 |
| Restrictive pattern | 32 (29%) | 24 (31%) | 8 (27%) | 0.815 |
| Characteristic | Overall Population (n = 109) | LV Remodeling Group (n = 79) | LV Nonremodeling Group (n = 30) | p Value |
|---|---|---|---|---|
| Biomarkers of STEMI | ||||
| Peak serum creatine kinase (U/L) | 2,318 ± 1961 | 2,146 ± 1,981 | 2,770 ± 1862 | 0.138 |
| Peak serum creatine kinase-MB (ng/ml) | 159 ± 101 | 155 ± 96 | 170 ± 113 | 0.505 |
| Peak serum lactate dehydrogenase (U/L) | 1,360 ± 811 | 1,259 ± 768 | 1,626 ± 871 | 0.034 |
| Peak serum troponin I (ng/ml) | 33 ± 31 | 28 ± 27 | 45 ± 36 | 0.011 |
| Peak serum myoglobin (ng/ml) | 809 ± 858 | 716 ± 706 | 1,054 ± 1,147 | 0.066 |
| Treatment of STEMI | ||||
| Thrombolysis plus PCI | 15 (14%) | 10 (13%) | 5 (17%) | 0.551 |
| PCI | 37 (34%) | 25 (32%) | 12 (40%) | 0.498 |
| Rescue PCI | 55 (51%) | 43 (54%) | 12 (40%) | 0.203 |
| Culprit lesion | ||||
| Left anterior ascending coronary artery | 63 (58%) | 43 (54%) | 20 (67%) | 0.283 |
| Left circumflex artery | 24 (22%) | 18 (23%) | 6 (20%) | 1 |
| Right coronary artery | 41 (38%) | 32 (41%) | 9 (30%) | 0.379 |
| Extension of coronary artery disease | ||||
| 1-vessel disease | 72 (66%) | 53 (67%) | 19 (65%) | 1 |
| 2-vessel disease | 26 (24%) | 17 (21%) | 9 (30%) | 0.451 |
| 3-vessel disease | 9 (8%) | 8 (10%) | 1 (3.4) | 0.439 |
| Hospital discharge therapy | ||||
| Antiplatelet drugs | 109 (100%) | 79 (100%) | 30 (100%) | NA |
| Angiotensin-converting enzyme inhibitors | 78 (72%) | 59 (75%) | 19 (63%) | 0.246 |
| Angiotensin receptor blockers | 4 (4%) | 4 (5%) | 1 (3%) | 0.574 |
| β blockers | 81 (74%) | 61 (77%) | 20 (67%) | 0.327 |
| Statins | 77 (71%) | 59 (75%) | 18 (60%) | 0.160 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


