Differences in enrollment criteria and protocol requirements are believed to affect patient representation and outcomes from premarket and postmarket surveillance (PMS) trials. These differences have not been assessed in studies evaluating coronary stenting. We aimed to assess differences in clinical profile and long-term outcomes in patients enrolled into premarket versus PMS trials assessing the Endeavor zotarolimus-eluting stent (E-ZES). We pooled patient-level data for 2,132 and 4,357 E-ZES–treated subjects enrolled into the ENDEAVOR program (premarket) and Patient Related OuTcomes with Endeavor versus Cypher stenting Trial (PMS), respectively. Follow-up data were available through 3 years. Baseline characteristics and outcomes of patients enrolled in the 2 groups were compared. Propensity score–adjusted Cox proportional hazards models were used to assess the effect of differences in baseline characteristics. We also adjusted for protocol-mandated repeat angiography to account for differences in follow-up requirements. Despite significant differences in baseline characteristics, the unadjusted 3-year rates of major adverse cardiac events, major adverse cardiac and cerebrovascular events, and target vessel failure were similar (premarket vs PMS: 11.9% vs 12.7%, p = 0.369; 12.7% vs 13.9%, p = 0.191; and 13.8% vs 13.4%, p = 0.667, respectively). However, PMS trials had significantly higher rates of myocardial infarctions (p = 0.005) and definite or probable stent thrombosis (p = 0.016). After propensity score adjustment, myocardial infarction rates remained significantly different (hazard ratio 0.53, 95% confidence interval 0.30 to 0.91). To conclude, premarket and PMS trials assessing E-ZES implantation enrolled different patients. PMS trials were shown to be essential for the detection of safety signals.
Although there is common agreement that differences between pre- and post-market studies exist, these observations have not been validated in trials assessing coronary artery stenting, and the differences are yet to be quantified. The objective of this study is to retrospectively assess the differences in patient representation and clinical outcomes between pre- and post-market trials assessing Endeavor zotarolimus-eluting stent (E-ZES) implantation and to evaluate whether postmarket surveillance (PMS) trials provide additional information on the efficacy and safety profile of these technologies.
Methods
The investigators sought and were granted access to deidentified patient-level data from 6 pre–Food and Drug Administration approval (premarket) trials (the ENDEAVOR PK [NCT00314275], ENDEAVOR I, ENDEAVOR II, ENDEAVOR II Continued Access Registry [CA], ENDEAVOR III, and ENDEAVOR IV trials) and 1 post–Food and Drug Administration approval trial (Patient Related OuTcomes with Endeavor versus Cypher stenting Trial ) assessing the Endeavor zotarolimus-eluting (E-ZES) stent. These were multicenter, prospective trials for which 3 to 5 years follow-up data are available. Details of study design and results for each study were previously reported.
Inclusion and exclusion criteria were similar across the premarket trials. Subjects were required to have clinical evidence of ischemic coronary disease or a positive functional study. Subjects presenting with ST-segment elevation myocardial infarction were excluded. Angiographic requirements were the presence of a single de novo native coronary lesion with a diameter stenosis of at least 50% but <100% by visual estimate. Reference vessel diameter had to be from 2.5 to 3.5 mm and lesion length range from 14 to 32 mm. In the PMS trial, subjects undergoing elective, unplanned, or emergency procedures in native coronary arteries were recruited if their indication, lesion length, and vessel diameter of target lesion(s) were in accordance with manufacturers’ indications and instructions for use. There were no restrictions on the number of vessels that could be treated. We included only E-ZES–treated subjects in the current analysis.
Outcomes were available for at least a 3-year follow-up period in all included studies. These included major adverse cardiac events (MACE; defined as a composite of all-cause death, myocardial infarction [MI], clinically driven [CD] target lesion revascularization [TLR], and emergent coronary artery bypass surgery), major adverse cardiac and cerebrovascular events (MACE plus stroke), and target vessel failure (TVF; defined as cardiac death, MI, and CD target vessel revascularization [TVR]). Other outcomes included stent thrombosis (ST) and the individual components of the TVF and major adverse cardiac and cerebrovascular events composites. Cardiac death included any death that was not clearly related to a noncardiac cause. MI was defined by total creatine kinase (CK) more than twice the individual clinical site laboratory normal. If CK data were missing, then CK-MB or troponin, in descending order of preference, >3 times the individual clinical site laboratory normal were used. CD TLR and TVR and definite or probable ST were defined according to recommendations of the Academic Research Consortium. All end points were adjudicated by the same independent clinical events committee.
Descriptive statistics are shown for demographics, co-morbidities, and angiographic characteristics. Continuous variables are presented as mean ± SD and discrete variables as percentage and were compared with a t test and a chi-square test, respectively. Unadjusted rate of clinical outcomes through 3 years are reported as Kaplan-Meier estimates and were compared using log-rank statistic. We first compared all baseline characteristics and unadjusted outcomes between the premarket and PMS groups. To determine whether differences in outcomes could be accounted for by differences in baseline characteristics, Cox proportional hazards models were used. This included a propensity score adjustment for membership in study category, given the baseline characteristics. The outcome variables were regressed on all the baseline characteristics with sufficient data. As higher rates of repeat revascularization are expected in studies in which repeat angiographic follow-up is mandated, end points, which include revascularizations, were also adjusted for protocol-mandated repeat angiography.
We conducted sensitivity analyses to assess the impact of repeat angiography and analyze if the differences in MI rates are driven by periprocedural events, by using landmark analyses at 270 days from revascularization and 48 hours for MI. All tests were assessed at a significance level of 0.05.
Results
The ENDEAVOR pooled cohort (premarket) and Patient Related OuTcomes with Endeavor versus Cypher stenting Trial (PMS) comprised 2,132 and 4,357 subjects treated with E-ZES, respectively. Many of the baseline clinical and angiographic characteristics differed between the 2 cohorts ( Table 1 ). The premarket group had a greater proportion of women and patients with history of hypertension, hyperlipidemia, previous MI, and family history of coronary artery disease, whereas the PMS group included more patients with history of smoking. More subjects enrolled in the premarket studies presented with unstable angina, compared with a significantly higher rate of presentation with MI in the postmarket trial. More lesions were treated per subject in the PMS trial (as only a single lesion was allowed in the premarket studies).
| Baseline Characteristics | Premarket (n = 2132) | Postmarket ∗ (n = 4357) | p-Value |
|---|---|---|---|
| Age (years) | 0.423 | ||
| Mean ± SD (N) | 62.5 ± 10.7 (2132) | 62.3 ± 10.6 (4357) | |
| Median | 63.0 | 62.0 | |
| Range (min,max) | (27.0,92.0) | (23.0,92.0) | |
| Men | 71.5% (1524/2132) | 76.7% (3340/4357) | <0.001 |
| Hyperlipidemia | 81.2% (1720/2118) | 61.8% (2694/4357) | <0.001 |
| Current smoker | 23.4% (491/2094) | 24.9% (1084/4357) | 0.210 |
| History of smoking | 49.2% (1035/2105) | 57.7% (2515/4357) | <0.001 |
| Prior myocardial infarction | 28.5% (604/2117) | 20.3% (884/4357) | <0.001 |
| Prior percutaneous coronary intervention | 26.0% (554/2130) | 12.3% (534/4357) | <0.001 |
| Prior coronary artery bypass | 6.7% (143/2132) | 4.6% (199/4357) | <0.001 |
| Hypertension | 73.0% (1551/2126) | 64.6% (2814/4357) | <0.001 |
| Premature coronary arterial disease in first-degree relative | 41.5% (744/1792) | 34.2% (1288/3768) | <0.001 |
| Diabetes mellitus | 26.1% (555/2129) | 26.9% (1174/4357) | 0.453 |
| Insulin treatment | 8.3% (158/1908) | 6.5% (285/4357) | 0.013 |
| Stable angina pectoris | 49.3% (909/1843) | 49.5% (2156/4357) | 0.907 |
| Acute coronary syndrome | 50.7% (934/1843) | 44.0% (1919/4357) | <0.001 |
| Unstable angina pectoris | 40.8% (752/1843) | 18.3% (796/4357) | <0.001 |
| MI at enrollment | 9.9% (182/1843) | 25.8% (1123/4357) | <0.001 |
| Type B2/C lesions | 71.4% (1516/2124) | 55.2% (2404/4355) | <0.001 |
| Ejection fraction (%) | 0.783 | ||
| Mean ± SD (N) | 58.9 ± 10.9 (1841) | 58.8 ± 12.6 (2236) | |
| Median | 60.0 | 60.0 | |
| Range (min,max) | (25.0,94.0) | (15.0,96.0) | |
| Reference vessel diameter (mm) | <0.001 | ||
| Mean ± SD (N) | 2.7 ± 0.5 (2124) | 3.0 ± 0.5 (4348) | |
| Median | 2.7 | 3.0 | |
| Range (min,max) | (1.6,5.1) | (1.1,5.2) | |
| Minimum lumen diameter (mm) | <0.001 | ||
| Mean ± SD (N) | 0.9 ± 0.4 (2124) | 0.4 ± 0.4 (4349) | |
| Median | 0.8 | 0.4 | |
| Range (min,max) | (0.0,2.6) | (0.0,2.4) | |
| Diameter stenosis (%) | <0.001 | ||
| Mean ± SD (N) | 67.4 ± 12.4 (2124) | 85.4 ± 11.9 (4352) | |
| Median | 68.2 | 90.0 | |
| Range (min,max) | (20.3,100.0) | (32.0,100.0) | |
| Calcification | 0.671 | ||
| Mild | 73.8% (1568/2124) | 72.8% (3172/4355) | |
| Moderate | 20.8% (442/2124) | 21.4% (933/4355) | |
| Severe | 5.4% (114/2124) | 5.7% (250/4355) | |
| Tortuosity | <0.001 | ||
| Mild | 88.2% (1871/2122) | 77.6% (3381/4355) | |
| Moderate | 9.7% (206/2122) | 19.9% (865/4355) | |
| Severe | 2.1% (45/2122) | 2.5% (109/4355) | |
| Thrombus | 2.7% (58/2124) | 9.4% (408/4355) | <0.001 |
| Lesion length (mm) | <0.001 | ||
| Mean ± SD (N) | 14.2 ± 6.1 (2110) | 18.1 ± 9.6 (4348) | |
| Median | 13.0 | 15.0 | |
| Range (min,max) | (3.0,52.4) | (1.0,95.0) | |
| Number of lesions treated per patient | <0.001 | ||
| Mean ± SD (N) | 1.0 ± 0.0 (2132) | 1.4 ± 0.7 (4355) | |
| Median | 1.0 | 1.0 | |
| Range (min,max) | (1.0,2.0) | (0.0,6.0) |
∗ Lesion characteristics in the postmarket trial are based on the lesion with the highest percent diameter stenosis, smallest MLD, and largest lesion length.
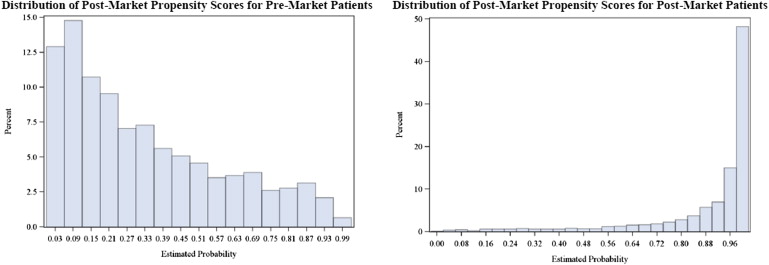
Figure 1 depicts marked differences in propensity score distribution between the premarket and PMS cohorts. Only a small proportion of the premarket cohort had propensity scores that overlapped with scores of the PMS cohort, signifying differences in patient selections into these 2 study types.
Unadjusted event rates of MACE, major adverse cardiac and cerebrovascular events, and TVF did not significantly differ between premarket studies and PMS (p = 0.369, p = 0.191, and p = 0.667, respectively). CD TVR occurred more often in the premarket studies (11.2% vs 8.4%, p <0.001), whereas MI and definite or probable ST occurred more often in the PMS study (2.8% vs 4.3%, p = 0.005, and 0.7% vs 1.5%, p = 0.016, respectively; Table 2 ).
| Outcome | Premarket (n = 2132) | Postmarket (n = 4357) | Unadjusted p Value ∗ | Adjusted Hazard Ratio (Post vs. Pre) | Adjusted p Value ∗ | ||
|---|---|---|---|---|---|---|---|
| n (%) | KM Estimate | n (%) | KM Estimate | ||||
| Major adverse cardiac and cerebrovascular event | 256 (12.6%) | 12.7% | 579 (13.9%) | 13.9% | 0.191 | 1.00 (0.74–1.35) | 0.980 |
| Major adverse cardiac events | 240 (11.8%) | 11.9% | 529 (12.7%) | 12.7% | 0.369 | 1.05 (0.77–1.43) | 0.746 |
| Target vessel failure | 278 (13.7%) | 13.8% | 556 (13.3%) | 13.4% | 0.667 | 1.16 (0.87–1.55) | 0.324 |
| Death | 71 (3.5%) | 3.5% | 181 (4.3%) | 4.3% | 0.133 | 0.76 (0.47–1.23) | 0.267 |
| Cardiac death | 26 (1.3%) | 1.3% | 109 (2.6%) | 2.6% | <0.001 | 0.60 (0.29–1.21) | 0.153 |
| Myocardial infarction | 57 (2.8%) | 2.8% | 179 (4.3%) | 4.3% | 0.005 | 0.53 (0.30–0.91) | 0.021 |
| Clinically driven target lesion revascularization | 139 (6.8%) | 6.9% | 238 (5.7%) | 5.8% | 0.077 | 1.28 (0.83–1.96) | 0.261 |
| Clinically driven target vessel revascularization | 224 (11.0%) | 11.2% | 348 (8.3%) | 8.4% | <0.001 | 1.45 (1.04–2.04) | 0.031 |
| Definite/probable stent thrombosis | 15 (0.7%) | 0.7% | 61 (1.5%) | 1.5% | 0.016 | 0.55 (0.23–1.30) | 0.174 |
∗ Unadjusted p-values are based on log rank statistics. Adjusted p-values are based on Cox proportional hazards model and are adjusted for propensity scores (death, MI, and ST) and propensity score and protocol mandated repeat angiography (MACCE, MACE, TVF, CD TVR, and CD TLR).
After adjusting for repeat angiography mandated by propensity scores and the protocol, the differences in revascularization and MI rates remained statistically significant (CD TVR, hazard ratio 1.45, 95% confidence interval 1.04 to 2.04, p = 0.031; MI, hazard ratio 0.53, 95% confidence interval 0.30 to 0.91, p = 0.021).
In a landmark analysis of CD TVR, differences were significant through 270 days of follow-up and nonsignificant thereafter (p = 0.021 and p = 0.220, respectively). In a landmark analysis of MI rates before and after 48 hours, periprocedural rates did not contribute to the overall differences between the 2 study cohorts (p = 0.390 and p = 0.008, respectively; Supplementary Table 1 and Supplementary Figures 1 and 2 ).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


