Bioprosthetic valve replacement is the treatment of choice in older patients with symptomatic severe aortic valve disease. Thrombosis of bioprosthetic valves has been considered a rare complication; however, in the presence of valvular obstruction, therapeutic consequences for the individual patient may be dramatic including repeat valve replacement or thrombolysis. We therefore evaluated oral anticoagulation with phenprocoumon as an alternative treatment for obstructive thrombosis of bioprosthetic valves. Six of 470 patients who had received a single stented bioprosthetic aortic valve from January 2007 through December 2008 at our hospital presented with obstructive bioprosthetic valve thrombosis within 14 months postoperatively. All 6 patients (1% of study population) had received a porcine valve (p = 0.1 vs pericardial), were hemodynamically stable, were in sinus rhythm, and were taking acetylsalicylic acid 100 mg/day. Echocardiography showed an increase in mean pressure gradient early postoperatively from 23.3 ± 4 to 57.0 ± 10 mm Hg (p <0.001). Five patients were started on phenprocoumon and followed for 114 ± 54 days, when mean pressure gradient had returned to 23.5 ± 6 mm Hg. No adverse events were observed during that period. One patient presenting with dyspnea and fever underwent emergency repeat valve replacement for suspected endocarditis, with histology showing long-term thrombosis of the explanted valve. In conclusion, oral anticoagulation with phenprocoumon represents a safe and effective treatment in clinically stable patients with obstructive thrombosis of bioprosthetic aortic valves, thus obviating repeat valve surgery or thrombolysis.
Thrombosis of bioprosthetic valves implanted in the aortic position is thought to be rare, with an incidence from 0.03% to 0.13% per year. In contrast, autopsy studies have demonstrated considerably higher rates of thrombosis up to 11% depending on valve type and position. Current guidelines and recommendations advocate reoperation or thrombolysis for prosthetic valve thrombosis associated with an increased transvalvular gradient (obstructive thrombosis). Type of prosthesis (mechanical vs bioprosthetic) is considered not to have particular therapeutic implications, although recommendations are largely based on data from mechanical valves. Therefore, treatment of obstructive thrombosis of bioprosthetic aortic valves as delineated in the limited number of published case reports predominantly consists of repeat valve replacement or thrombolysis. The 2 treatment options carry considerable risk and in clinically stable patients (New York Heart Association classes I to III) may appear disproportionate. We therefore investigated oral anticoagulation with phenprocoumon as an alternative treatment for obstructive thrombosis of bioprosthetic aortic valves in hemodynamically stable patients.
Methods
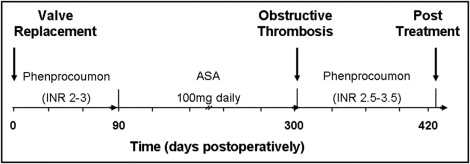
From January 2007 through December 2008, 470 patients received a single stented bioprosthetic aortic valve at our institution. Four types of stented bioprosthesis (porcine Epic, St. Jude Medical, St. Paul, Minnesota; porcine Mosaic, Medtronic, Minneapolis, Minnesota; pericardial Perimount Magna, Edwards Lifesciences, Irvine, California; pericardial Mitroflow, Sorin, Milano, Italy) were implanted during that period. Patients with double-valve or transapical implantation were excluded from the analysis as were patients who received a nonstented valve. Institutional policy for postoperative antithrombotic therapy was 3 months of a vitamin K antagonist (phenprocoumon) with an international normalized ratio of 2.5 to 3.0 followed by acetylsalicylic acid 100 mg/day. After hospital discharge, patients were seen regularly by their treating physician and referred for repeat cardiologic assessment only when deemed necessary. In 6 patients referred back to our hospital for various reasons, a second transthoracic echocardiography was suggestive of obstructive thrombosis. Transesophageal echocardiography (TEE) was then performed under mild sedation using a Philips ie33 echocardiograph (Philips, Hamburg, Germany) with a multiplane 7-MHz transducer. Five of the 6 patients with obstructive thrombosis of the bioprosthetic aortic valve were considered hemodynamically stable (systolic blood pressure >100 mm Hg, pulse 60 to 90 beats/min, New York Heart Association classes I to III, no clinical or radiologic signs of heart failure) and started on unfractionated heparin (partial thromboplastin time 60 to 80) and subsequently on oral anticoagulation with phenprocoumon (target international normalized ratio 2.5 to 3.5) and followed prospectively for 3 months, when they were seen for clinical and echocardiographic follow-ups ( Figure 1 ) .
Echocardiography was performed according to guidelines for the clinical application of echocardiography. Details have been published previously. Briefly, maximal flow velocity within the aortic valve prosthesis (v 2 ) was recorded by aligning the continuous-wave beam parallel to the aortic jet using the best of multiple windows. The velocity curve was traced and mean pressure gradient was calculated automatically from the mean of a series of instantaneous velocities of a single beat measured during the systolic ejection period using the simplified Bernoulli equation (ΔPm = Σ4 × [v i ] 2 ), where v i is instantaneous velocity. Flow velocity in the left ventricular outflow tract (v 1 ) was measured by pulse-wave Doppler just below the aortic valve. The sample volume was placed 1 cm below the aortic valve and then slowly moved toward the valve until an increase in velocity and spectral broadening was seen. Thereafter, the sample volume was moved back until a narrow band of flow velocity was obtained. Flow velocity in the aortic valve v 2 was obtained from continuous-wave Doppler of the aortic jet. The relation of v 1 to v 2 (dimensionless index of velocities) was first calculated and also obtained from repeated continuous-wave Doppler signals with simultaneous visualization of the maximum velocity of the valve v 2 and the left ventricular outflow tract v 1 , if possible.
Statistical analysis was performed using SPSS 15.0 (SPSS, Inc., Chicago, Illinois). Continuous variables are presented as mean ± SD and categorical variables as percentage. Continuous variables were compared by Student’s t test and categorical variables by chi-square analysis or with Fisher’s exact test. A p value <0.05 was considered statistically significant.
Results
Of the 470 stented bioprosthetic aortic valves implanted in 2007 and 2008, 81 were porcine Epic, 227 porcine Mosaic, 156 pericardial Perimount, and 6 pericardial Mitroflow valves. Concomitant bypass surgery was performed in 31% of patients (147 of 470). Thirty-day mortality was 6% (28 of 470). Baseline clinical, surgical, and early postoperative echocardiographic parameters are presented in Table 1 .
| Variable | Bioprosthetic Thrombosis | p Value | |
|---|---|---|---|
| Yes (n = 6) | No (n = 464) | ||
| Age (years) | 69.7 ± 8 | 75.7 ± 7 | 0.03 |
| Women | 2 (33%) | 205 (44%) | 0.70 |
| Height (cm) | 170.3 ± 8 | 167.3 ± 9 | 0.42 |
| Weight (kg) | 78.0 ± 11 | 77.1 ± 14 | 0.88 |
| Body surface area (m 2 ) | 1.9 ± 0.2 | 1.9 ± 0.2 | 0.71 |
| Body mass index (kg/m 2 ) | 27.0 ± 4 | 27.5 ± 4 | 0.77 |
| Porcine bioprosthesis | 6 (100%) | 302 (65%) | 0.10 |
| Prosthesis size (mm) | 22.0 ± 2 | 22.1 ± 2 | 0.83 |
| Operation time (minutes) | 189 ± 82 | 172 ± 46 | 0.40 |
| Postoperative echocardiography | |||
| Left ventricular end-diastolic diameter (mm) | 51.5 ± 3 | 50.2 ± 8 | 0.68 |
| Left ventricular end-systolic diameter (mm) | 33.8 ± 5 | 32.8 ± 9 | 0.80 |
| Fractional shortening (%) | 34.8 ± 7 | 35.6 ± 10 | 0.87 |
| Bioprosthesis | |||
| Peak velocity (m/s) | 3.3 ± 0.3 | 2.8 ± 0.5 | 0.01 |
| Mean pressure gradient (mm Hg) | 23.3 ± 4 | 17.0 ± 7 | 0.02 |
| Dimensionless index | 0.31 ± 0.01 | 0.40 ± 0.03 | 0.01 |
| Effective orifice area index (cm 2 /m 2 ) | 0.66 ± 0.14 | 0.71 ± 0.10 | 0.26 |
Six of 470 patients (1%) were diagnosed with obstructive thrombosis on average 318 ± 72 days postoperatively. On presentation all patients were in sinus rhythm, taking acetylsalicylic acid 100 mg/day, and hemodynamically stable with a systolic blood pressure of 135 ± 7 mm Hg, diastolic blood pressure 79 ± 7 mm Hg, and a pulse of 65 ± 10 beats/min. Two complained of dyspnea, 1 had developed a transitory ischemic attack, and 2 were asymptomatic but had been diagnosed with an increased transvalvular gradient by routine transthoracic echocardiography (TTE; Table 2 ). TTE showed an increase in mean pressure gradient in all 6 patients (postoperative 23.3 ± 4 to 57.0 ± 10 mm Hg, p <0.001; Table 3 , Figure 2 ) , and the dimensionless index decreased from 0.31 ± 0.01 to 0.21 ± 0.03. Risk factors for thrombosis in these patients were previous cerebrovascular accident in 1, paroxysmal atrial fibrillation in 1, and peripheral vascular disease in 2. All had normal left ventricular systolic function and none had current or previous cancer or previous endocarditis. Compared to patients without thrombosis the 6 patients with thrombosis were younger and had higher postoperative peak velocity and mean pressure gradient. All other parameters were comparable between groups ( Table 1 ). All patients with obstructive thrombosis had received porcine valves, 2 Epic and 4 Mosaic, which did not reach statistical significance compared to patients with pericardial valves.
| Baseline | Event | After Treatment ⁎ | ||||||
|---|---|---|---|---|---|---|---|---|
| Patient Number | Age (years)/Sex | Operation | Size (mm) | NYHA Class | Days to Symptoms | NYHA Class | Days to Follow-Up | |
| Year | Valve Type | |||||||
| 1 | 64/M | 2007 | Mosaic | 25 | I | 309 | I | 110 |
| 2 | 76/M | 2007 | Mosaic | 21 | III | 244 | III † | 103 |
| 3 | 81/F | 2007 | Epic | 19 | III | 425 | III † | 60 |
| 4 | 63/M | 2008 | Mosaic | 25 | I | 371 | I | 92 |
| 5 | 63/M | 2008 | Epic | 21 | I | 240 | I | 205 |
| 6 | 71/F | 2008 | Mosaic | 21 | II | 317 | I ‡ | N/A |
⁎ After treatment reflects status after 102 ± 49 days of phenprocoumon (target rate 2.5 to 3.5).
† Patient 2 was subsequently diagnosed with lung cancer and patient 3 with grade IV diastolic dysfunction.
‡ Patient 6 underwent emergency repeat valve replacement for suspected infective endocarditis.
| After Operation | Event | After Treatment | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patient Number | V max (m/s) . | MPG (mm Hg) | DVI | V max (m/s) . | MPG (mm Hg) | DVI | TEE | V max (m/s) . | MPG (mm Hg) | DVI |
| 1 | 3.4 | 22 | 0.29 | 4.5 | 51 | 0.21 | Not performed | 3.6 | 26 | 0.29 |
| 2 | 3.1 | 22 | 0.32 | 4.7 | 49 | 0.24 | Nondiagnostic | 3.4 | 24 | 0.37 |
| 3 | 3.1 | 22 | 0.32 | 4.9 | 57 | 0.23 | Nondiagnostic | 3.6 | 34 | 0.33 |
| 4 | 3.1 | 20 | 0.29 | 4.5 | 51 | 0.20 | Thickened cusp, transvalvular AR | 3.0 | 20 | 0.34 |
| 5 | 3.6 | 24 | 0.29 | 4.9 | 59 | 0.20 | Transvalvular AR | 2.9 | 21 | 0.33 |
| 6 | 3.7 | 30 | 0.33 | 5.5 | 75 | 0.19 | Thickened cusps | 2.5 | 16 | 0.43 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


