Neutrophil/lymphocyte ratio (NLR) is the strongest white blood cell predictor of adverse outcomes in stable and unstable coronary artery syndromes. The aim of our study was to explore the utility of NLR in predicting long-term mortality in patients with non–ST-segment elevation myocardial infarction (NSTEMI). Consecutive patients with NSTEMI at Staten Island University Hospital were evaluated for study inclusion. Of the 1,345 patients with NSTEMI admitted from September 2004 to September 2006, 619 qualified for study inclusion. Survival analysis, stratified by NLR tertiles, was used to evaluate the predictive value of average inpatient NLR levels. Four-year vital status was accessed with electronic medical records and Social Security Death Index. Patients in the highest NLR tertile (NLR >4.7) had a higher 4-year mortality rate (29.8% vs 8.4%) compared to those in the lowest tertile (NLR <3, Wilcoxon chi-square 34.64, p <0.0001). After controlling for Global Registry of Acute Coronary Events risk profile scores, average NLR level remained a significant predictor of inpatient and 4-year mortality. Hazard ratios per unit increase of average NLR (log) increased by 1.06 (p = 0.0133) and 1.09 (p = 0.0006), respectively. In conclusion, NLR is an independent predictor of short-term and long-term mortalities in patients with NSTEMI with an average NLR >4.7. We strongly suggest the use of NLR rather than other leukocyte parameters (e.g., total white blood cell count) in risk stratification of the NSTEMI population.
Many studies have evaluated the relation between white blood cell subtypes and adverse coronary artery outcomes. Of all leukocyte parameters, neutrophil/lymphocyte ratio (NLR) was the strongest predictor of adverse outcomes in patients who underwent coronary bypass graft surgery or percutaneous coronary intervention. NLR predicts long-term mortality in ST-segment elevation myocardial infarction and in-hospital and 6-month mortalities in acute coronary syndromes and is an independent predictor for cardiac mortality in stable coronary artery disease. Although the relation between NLR and adverse outcomes in stable and unstable coronary syndromes has been studied, previous studies have not focused on the relation between NLR and long-term mortality in patients with non–ST-elevation myocardial infarction (NSTEMI). The aim of our study was to investigate the usefulness of NLR during hospitalization in predicting long-term mortality in patients with NSTEMI.
Methods
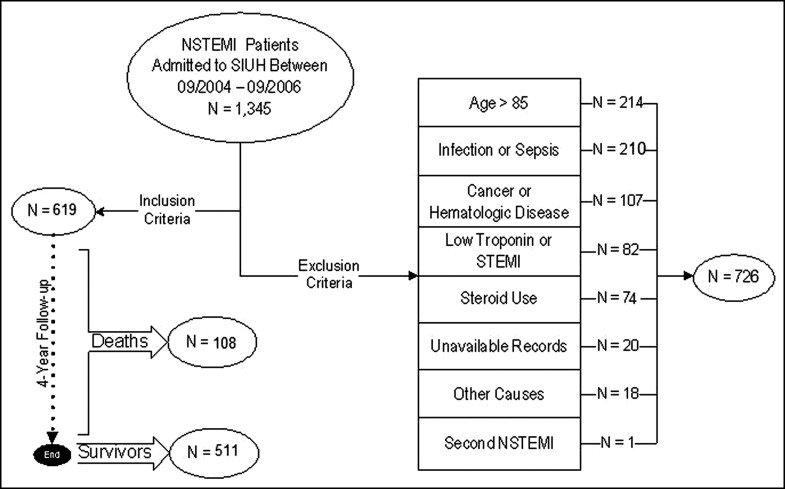
This retrospective longitudinal observational study explored the predictive value of NLR on short- and long-term survival in 1,345 patients with NSTEMI discharged from Staten Island University Hospital (Staten Island, New York), the only angioplasty-providing tertiary center in Staten Island, with a semi-isolated population of 443,728 (77.6% white Americans), from September 2004 to September 2006. Study inclusion required a cardiologist-confirmed NSTEMI diagnosis with a documented gradual increase and decrease of serum troponin levels with a peak value >0.5 ng/ml. Exclusion criteria included age >85 years, clinical evidence of active infection, active cancer, hematologic proliferative diseases, active or chronic inflammatory or autoimmune diseases, steroid therapy or chemotherapy around the index diagnosis, negative cardiac enzymes, concomitant STEMI or unavailable complete blood cell count or medical records ( Figure 1 ). Of the 1,345 patients, only 619 were eligible for study inclusion. The primary end point, all-cause 4-year mortality, was obtained from electronic medical records and the Social Security Death Index.
White blood cell, neutrophil, lymphocyte, and monocyte absolute counts were obtained from the initial (within 1 hour of admission), second, and last blood cell counts in the same index hospitalization of >94% of patients (n = 586). Differential leukocyte counts were obtained by the Coulter Counter technique (Coulter Gen.S Hematology Analyzer, Beckman Coulter Corp., Hileh, Florida). Two physicians independently reviewed electronic medical records for potential confounders, including demographic variables, coronary artery disease risk factors, clinical prognostic risk factors at presentation, peak serum troponin and lowest serum creatinine levels, prescribed medications at discharge, laboratory values, revascularization procedures, left ventricular ejection fraction, and smoking history ( Table 1 ). We obtained left ventricular ejection fraction from echocardiographic reports. Smoking status included current and previous smoking. Discharge and admission Global Registry of Acute Coronary Events (GRACE) scores were calculated for each eligible index case. GRACE score was used because of its superiority and validity to predict short- and long-term mortality.
| NLR <3 (n = 203) | 3.0 ≤ NLR ≤4.7 (n = 208) | NLR >4.7 (n = 208) | p Value for Trend | |
|---|---|---|---|---|
| Variables | ||||
| Age (years) | 60.20 ± 0.86 | 64.76 ± 0.85 | 69.24 ± 0.80 | <0.0001 |
| Men | 125 (61.6%) | 145 (69.7%) | 147 (71.0%) | 0.0350 |
| Race (white) | 171 (89.1%) | 182 (93.8%) | 188 (97.4%) | 0.0009 |
| Body mass index (kg/m 2 ) | 30.19 ± 0.42 | 28.52 ± 0.41 | 28.70 ± 0.42 | 0.0086 |
| Previous cerebrovascular event | 10 (4.9%) | 12 (5.8%) | 19 (9.2%) | 0.0963 |
| Previous heart failure | 22 (10.8%) | 35 (16.8%) | 59 (28.5%) | <0.0001 |
| Previous coronary bypass | 23 (11.3%) | 39 (18.8%) | 47 (22.7%) | 0.0023 |
| Previous angioplasty | 44 (21.7%) | 42 (20.2%) | 43 (20.8%) | 0.8016 |
| Hypertension | 133 (65.5%) | 147 (70.7%) | 162 (78.3%) | 0.0049 |
| Diabetes mellitus | 58 (28.6%) | 73 (35.1%) | 86 (41.6%) | 0.0062 |
| End-stage renal disease | 4 (2.0%) | 6 (2.9%) | 8 (3.9%) | 0.2578 |
| Myocardial infarction | 56 (27.6%) | 68 (32.7%) | 68 (32.9%) | 0.2287 |
| Smoking | 128 (63.1%) | 112 (53.9%) | 108 (52.2%) | 0.0219 |
| Presentation | ||||
| ST-segment deviation | 55 (27.1%) | 81 (38.9%) | 82 (39.8%) | 0.0051 |
| Atrial fibrillation | 10 (5.0%) | 15 (7.2%) | 27 (13.0%) | 0.0043 |
| Killip class III or IV | 4 (2.0%) | 8 (3.9%) | 16 (7.7%) | <0.0001 |
| Ejection fraction (%) | 48.28 ± 0.77 | 45.41 ± 0.91 | 42.47 ± 0.99 | <0.0001 |
| Diastolic blood pressure | 76.51 ± 1.14 | 76.40 ± 1.18 | 72.70 ± 1.21 | 0.0345 |
| Systolic blood pressure | 142.14 ± 1.91 | 142.46 ± 1.96 | 139.45 ± 1.99 | 0.4874 |
| Heart rate | 75.58 ± 1.29 | 79.95 ± 1.42 | 83.86 ± 1.61 | 0.0003 |
| Global Registry of Acute Coronary Events score | ||||
| On admission | 113.18 ± 1.79 | 126.60 ± 2.22 | 144.83 ± 2.62 | <0.0001 |
| At discharge | 117.98 ± 1.90 | 130.18 ± 2.26 | 145.89 ± 2.46 | <0.0001 |
| In-hospital management | ||||
| Aspirin | 200 (98.5%) | 200 (96.2%) | 190 (91.8%) | 0.0013 |
| β blockers | 190 (93.6) | 188 (90.4%) | 183 (88.4%) | 0.0674 |
| Clopidogrel | 169 (83.3%) | 159 (76.4%) | 140 (67.6%) | 0.0003 |
| Statins | 176 (86.7%) | 176 (84.6%) | 173 (84.0%) | 0.4295 |
| Angiotensin convertase inhibitor | 83 (40.9%) | 84 (40.4%) | 91 (44.0%) | 0.5605 |
| Angiotensin receptor blocker | 24 (11.8%) | 26 (12.5%) | 28 (13.6%) | 0.5973 |
| Warfarin | 8 (3.9%) | 19 (9.1%) | 31 (15.0%) | 0.0002 |
| Coronary graft bypass | 33 (16.3%) | 52 (25.0) | 64 (29.9%) | 0.0010 |
| Angioplasty | 118 (58.1%) | 103 (49.5%) | 64 (30.1%) | <0.0001 |
| In-hospital laboratory data | ||||
| Serum creatinine (mg/dl) | 1.06 ± 0.03 | 1.23 ± 0.07 | 1.56 ± 0.09 | <0.0001 |
| Serum glucose (mg/dl) | 141.37 ± 4.67 | 154.37 ± 5.15 | 172.36 ± 7.2 | 0.0009 |
| High-density lipoprotein (mg/dl) | 36.57 ± 0.94 | 37.58 ± 1.17 | 36.55 ± 0.93 | 0.7199 |
| Low-density lipoprotein (mg/dl) | 115.59 ± 3.54 | 113.64 ± 3.4 | 105.31 ± 3.66 | 0.1016 |
| Peak troponin (ng/ml) | 5.94 ± 0.77 | 7.96 ± 1.21 | 11.75 ± 1.61 | 0.0041 |
| First white blood cell count (× 10/ 3 μl) | 8.35 ± 2.61 | 8.95 ± 2.75 | 10.62 ± 3.62 | <0.0001 |
| First lymphocyte count (× 10/ 3 μl) | 2.41 ± 0.07 | 1.85 ± 0.06 | 1.23 ± 0.04 | <0.0001 |
| First monocyte count (× 10/ 3 μl) | 0.62 ± 0.02 | 0.66 ± 0.02 | 0.68 ± 0.02 | 0.1669 |
| First neutrophil count (× 10/ 3 μl) | 5.03 ± 0.13 | 6.24 ± 0.15 | 8.52 ± 0.24 | <0.0001 |
| Neutrophil/lymphocyte ratio ⁎ | 2.25 ± 0.06 | 3.7 ± 0.1 | 8.82 ± 0.46 | <0.0001 |
⁎ Indicates the average of first, second, and last measured ratios.
The study was powered to evaluate the primary outcome with an estimated 7% 4-year mortality rate (16% vs 9%) difference between patients with NSTEMI in the highest and lowest NLR tertiles. To detect this effect with 80% power and a 5% type 1 error required a minimum of 184 cases in each tertile. To increase study comparability with previous studies and because the mortality distribution differed among cases with NLR >4.7 and <4.7, all analyses were stratified by average NLR tertiles (NLR <3.0, NLR 3.0 to ≤4.7, NLR >4.7). Distributions of continuous and categorical variables were presented as means ± SDs and frequencies and percentages, respectively. Group comparisons used chi-square analysis for categorical data. Continuous variables were compared to analysis of variance or Kruskal-Wallis analysis of ranks, depending on the probability distribution of the variable. All probabilities were 2-sided and p values <0.05 were considered statistically significant. Kaplan-Meier product-limit curves were used to illustrate 4-year mortality differences by NLR tertiles and Wilcoxon tests were used to evaluate the equality of the survivorship function across NLR tertiles.
Cox proportional hazards models, using the exact method, assessed the independent association of average NLR level with mortality. NLRs were left skewed and normalized with a logarithmic transformation. Violations of the constant hazard assumption were evaluated using the interaction between the covariate and time and by evaluation of log-log survivor plots. Influential statistics were evaluated with Schoenfeld residuals. Depending on the model, 0 outlier to 6 outliers were excluded. Predictor inclusion, in the multivariate Cox model, was based on score and best selection criteria and on partial likelihood ratio and multicollinearity tests. Unadjusted models, GRACE score-adjusted model, and confounding baseline characteristic-adjusted model were developed for inpatient and outpatient survival ( Table 2 ).
| Average NLR ⁎ | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| <3.0 | 3.0–4.7 | >4.7 | |||||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Inpatient mortality | |||||||||
| Unadjusted | 1.13 | 0.50–3.37 | 0.5906 | 2.14 | 1.01–4.53 | 0.0458 | 1.10 | 1.06–1.15 | <0.0001 |
| Adjusted | |||||||||
| Global Registry of Acute Coronary Events score | 1.16 | 0.45–3.72 | 0.7314 | 1.97 | 0.86–4.54 | 0.1089 | 1.06 | 1.01–1.10 | 0.0133 |
| Baseline characteristics † | 1.27 | 0.33–4.94 | 0.7312 | 2.11 | 0.86–5.15 | 0.1010 | 1.27 | 1.15–1.40 | <0.0001 |
| 4-Year mortality | |||||||||
| Unadjusted | 1.38 | 0.49–3.87 | 0.5382 | 2.41 | 1.11–5.29 | 0.0269 | 1.12 | 1.07–1.18 | <0.0001 |
| Adjusted | |||||||||
| Global Registry of Acute Coronary Events score | 1.22 | 0.49–3.05 | 0.6694 | 2.01 | 0.89–4.52 | 0.0922 | 1.09 | 1.04–1.16 | 0.0006 |
| Baseline characteristics ‡ | 1.49 | 0.56–3.96 | 0.4257 | 1.68 | 0.75–3.78 | 0.2076 | 1.09 | 1.04–1.14 | 0.0005 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


