A high heart rate (HR) predicts future cardiovascular events. We explored the predictive value of HR in patients with high-risk hypertension and examined whether blood pressure reduction modifies this association. The participants were 15,193 patients with hypertension enrolled in the Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial and followed up for 5 years. The HR was assessed from electrocardiographic recordings obtained annually throughout the study period. The primary end point was the interval to cardiac events. After adjustment for confounders, the hazard ratio of the composite cardiac primary end point for a 10-beats/min of the baseline HR increment was 1.16 (95% confidence interval 1.12 to 1.20). Compared to the lowest HR quintile, the adjusted hazard ratio in the highest quintile was 1.73 (95% confidence interval 1.46 to 2.04). Compared to the pooled lower quintiles of baseline HR, the annual incidence of primary end point in the top baseline quintile was greater in each of the 5 study years (all p <0.05). The adjusted hazard ratio for the primary end point in the highest in-trial HR heart rate quintile versus the lowest quintile was 1.53 (95% confidence interval 1.26 to 1.85). The incidence of primary end points in the highest in-trial HR group compared to the pooled 4 lower quintiles was 53% greater in patients with well-controlled blood pressure (p <0.001) and 34% greater in those with uncontrolled blood pressure (p = 0.002). In conclusion, an increased HR is a long-term predictor of cardiovascular events in patients with high-risk hypertension. This effect was not modified by good blood pressure control. It is not yet known whether a therapeutic reduction of HR would improve cardiovascular prognosis.
A fast heart rate (HR) is a strong predictor of adverse subsequent cardiovascular events. In the present analysis of the previously completed Valsartan Antihypertensive Long-term Use Evaluation (VALUE) trial, we explored 4 key issues. First, whether a greater baseline HR is associated with adverse outcomes in a group of patients selected for both hypertension and additional cardiovascular risk factors. Second, whether the increased risk associated with a fast baseline HR remained significant after adjustment for baseline blood pressure (BP) levels and other risk factors. Third, whether the risk of a high baseline HR would persist across the full 5-year duration of the VALUE trial. Finally, whether effective BP control decreases or abolishes the residual cardiovascular risk attributable to in-trial tachycardia.
Methods
VALUE was an investigator-designed, prospective, multinational, double-blind, randomized, active-controlled, parallel-group trial. The primary objective was, for the same level of achieved BP, to compare the long-term effects of valsartan and amlodipine-based antihypertensive therapies on the incidence of fatal and nonfatal cardiac events in patients with hypertension at high cardiovascular risk. The study design and main results have been previously published. The VALUE trial included patients who at baseline had additional cardiovascular risk factors or a history of cardiovascular diseases. On enrollment, 15,245 study participants were switched to the VALUE algorithm of a blinded 5-step intensification of treatment to reach the target BP of <140/90 mm Hg. The titration had to be completed within 6 months after enrollment. The primary end point was the interval to the first composite cardiac event. Stroke (fatal and nonfatal) was considered and analyzed as a secondary event. An analysis of all-cause mortality was also prespecified.
In the present preplanned secondary analysis of the VALUE data, we evaluated all outcomes as prespecified in the original VALUE study analytic plan. We detected a strong digit preference in the distribution of the clinic HR values. Consequently, in the present report, we analyzed the HRs obtained from electrocardiographic (ECG) recordings taken with the patient in the recumbent position at enrollment and annually throughout the trial. The ECG recordings were managed by an independent clinical research organization (Premier Research Worldwide, Peterborough Cambridgeshire, United Kingdom) and were analyzed from dedicated rhythm strips in 1 of the 2 reading centers (United States and United Kingdom).
We first explored whether the baseline HR predicts adverse cardiovascular outcomes in patients with established hypertension who had additional cardiovascular risk or disease factors according to an algorithm that included age and gender. Next, we evaluated whether the association remained significant after adjustments for baseline BP and the risk or disease factors. The trends detected for baseline HR were illustrated by hazard ratios comparing outcomes in each of the higher 4 quintiles to the lowest HR quintile. Thereafter, we assessed the persistence of the effect of the baseline HR by comparing the outcome rates in the highest HR quintile to the average rates in the lower 4 quintiles, separately for each of the 5 study years. Finally to assess whether in-trial BP lowering reduced or abolished the HR effect, we evaluated the effect of the HR at year 1. At that point of the study, the BP lowering had reached the nadir, and we compared the effect of in-trial HR on subsequent cardiovascular events in patients whose BP was controlled (≤139/89 mm Hg) or not controlled (≥140/≥90 mm Hg).
Statistical analyses were done using the intention-to-treat principle, with the exception that we excluded 52 participants (0.34% of total population) from all analyses. Of these 52 participants, 32 (0.21%) did not have baseline ECG records, 11 (0.07%) had a HR <40 beats/min, and in 9 (0.06%) the HR was >140 beats/min. We assumed that the extremely low HR rates might have reflected concomitant medication with β blockers and that the extreme tachycardia might be have resulted from drugs that increase the HR or from the presence of other acute conditions such as anemia, fever, hyperthyroidism, or subclinical heart failure. The elimination of 52 study participants reduced the number of primary end points from 1,599 in the original study to 1,588 in the present report. The baseline and in-trial HR quintiles were compared using 95% confidence intervals and a 2-sided significance level of 5%. Significance was assessed using the chi-square test for categorical variables and the F test for continuous variables. Cox regression analysis was used to investigate the association between the interval to cardiovascular events and the linear HR, HR quintiles, and the highest versus the pooled lower 4 HR quintiles. Additionally, covariate adjustments for baseline BP, age, gender, and numerous risk factors were performed. We used SAS, version 9.2 (TS1M0) (SAS Institute, Cary, North Carolina) under AIX, version 6.1 (IMB, Armonk, New York), for all analyses in the present report.
Results
In the Cox model adjusted for potential confounders, a 10-beats/min increase in the baseline HR was associated with a significant increase in the risk of the primary end point, heart failure, sudden deaths, myocardial infarction, stroke, and all-causes mortality ( Table 1 ). We also analyzed whether the use of β blockers affected the results. Among the patients not taking β blockers (n = 10,148), the associations of HR with outcome remained highly significant, with the exception of stroke ( Table 1 ). To illustrate the associations seen in Table 1 , we divided the study population into quintiles of baseline HR. The patient characteristics stratified by quintiles of baseline HR are listed in Table 2 . The percentage of women, body mass index, baseline BP, diabetes mellitus, and proteinuria tended to be greater in the upper HR quintile groups. In contrast, the percentage of patients with a history of coronary heart disease or taking β blockers was greatest in the lowest HR quintile and decreased in the higher quintiles. The boundary for the upper baseline HR quintile was ≥79 beats/min.
| Model | Primary Composite End Point | Heart Failure | Sudden Cardiac Death | Myocardial Infarction | Stroke | All-Cause Death |
|---|---|---|---|---|---|---|
| 1. Unadjusted | ||||||
| Hazard ratio | 1.16 | 1.25 | 1.20 | 1.09 | 1.12 | 1.20 |
| 95% Confidence interval | 1.12–1.20 | 1.19–1.31 | 1.12–1.30 | 1.04–1.16 | 1.05–1.18 | 1.16–1.25 |
| p Value | <0.0001 | <0.0001 | <0.0001 | 0.002 | 0.0003 | <0.0001 |
| 2. Adjusted for baseline BP † | ||||||
| Hazard ratio | 1.17 | 1.27 | 1.20 | 1.10 | 1.11 | 1.21 |
| 95% Confidence interval | 1.13–1.22 | 1.21–1.34 | 1.12–1.30 | 1.04–1.17 | 1.05–1.18 | 1.17–1.25 |
| p Value | <0.0001 | <0.0001 | <0.0001 | 0.0005 | 0.0003 | <0.0001 |
| 3. Adjusted for baseline BP † and risk factors ‡ | ||||||
| Hazard ratio | 1.16 | 1.24 | 1.18 | 1.10 | 1.09 | 1.19 |
| 95% Confidence interval | 1.12–1.20 | 1.18–1.30 | 1.10–1.28 | 1.04–1.27 | 1.03–1.16 | 1.15–1.23 |
| p Value | <0.0001 | <0.0001 | <0.0001 | 0.0009 | 0.005 | <0.0001 |
| 3. Adjusted for patients not taking β blockers | ||||||
| Hazard ratio | 1.16 | 1.24 | 1.17 | 1.09 | 1.07 | 1.20 |
| 95% Confidence interval | 1.11–1.21 | 1.17–1.31 | 1.07–1.28 | 1.01–1.17 | 1.00–1.15 | 1.15–1.25 |
| p Value | <0.0001 | <0.0001 | 0.0009 | 0.02 | 0.06 | <0.0001 |
⁎ Hazard ratios and 95% confidence intervals reflect risk associated with 10-beats/min increase in heart rate.
† Systolic and diastolic blood pressure.
‡ Age, gender, race, body mass index, total cholesterol, smoking, diabetes mellitus, history of coronary heart disease, cerebrovascular disease, or peripheral arterial disease, left ventricular hypertrophy, and use of β blockers, calcium antagonists, or other antihypertensive drugs.
| Variable | Quintile | p Value | ||||
|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | ||
| Patients (n) | 2,832 | 2,775 | 3,396 | 2,891 | 3,299 | — |
| Age (years) | 67.1 ± 7.9 | 67.2 ± 8.0 | 67.3 ± 8.1 | 67.2 ± 8.2 | 67.4 ± 8.4 | 0.78 |
| Baseline heart rate (beats/min) | 55.5 ± 6.4 | 63.0 ± 6.9 | 67.9 ± 7.3 | 73.9 ± 8.8 | 85.0 ± 11.7 | — |
| Heart rate quintile interval (beats/min) | 40.0–57.0 | 58.0–63.0 | 64.0–70.0 | 71.0–78.0 | 79.0–139.0 | — |
| Women | 859 (30.3%) | 1,080 (38.9%) | 1,516 (44.6%) | 1,385 (47.9%) | 1,609 (48.8%) | <0.0001 |
| White | 2,570 (90.7%) | 2,524 (91.0%) | 3,030 (89.2%) | 2,580 (89.2%) | 2,870 (87.0%) | <0.0001 |
| Body mass index (kg/m 2 ) | 28.0 (4.2) | 28.4 (4.7) | 28.7 (4.9) | 28.9 (5.3) | 29.1 (5.8) | <0.0001 |
| Baseline diastolic blood pressure (mm Hg) | 85.5 (10.9) | 86.9 (10.5) | 87.4 (10.8) | 88.2 (10.7) | 89.3 (10.7) | <0.0001 |
| Baseline systolic blood pressure (mm Hg) | 153 (19.6) | 154 (18.8) | 154 (18.6) | 155 (18.9) | 157 (18.9) | <0.0001 |
| Antihypertensive drugs at baseline | 2,703 (95.5%) | 2,601 (93.9%) | 3,147 (92.7%) | 2,660 (92.1%) | 2,999 (91.1%) | <0.0001 |
| β Blockers | 1,550 (54.8%) | 1,157 (41.8%) | 1,045 (30.8%) | 707 (24.5%) | 586 (17.8%) | <0.0001 |
| Coronary heart disease | 1,619 (57.2%) | 1,395 (50.3%) | 1,528 (45.0%) | 1,167 (40.4%) | 1,246 (37.8%) | <0.0001 |
| Peripheral arterial disease | 352 (12.4%) | 380 (13.7%) | 497 (14.6%) | 417 (14.4%) | 459 (13.9%) | 0.08 |
| Stroke or transient ischemic attack | 569 (20.1%) | 529 (19.1%) | 674 (19.8%) | 586 (20.3%) | 648 (19.6%) | 0.90 |
| Left ventricular hypertrophy with strain pattern | 195 (6.9%) | 174 (6.3%) | 198 (5.8%) | 156 (5.4%) | 186 (5.6%) | 0.017 |
| Diabetes mellitus | 544 (19.2%) | 705 (25.4%) | 1,023 (30.1%) | 1,032 (35.7%) | 1,498 (45.4%) | <0.0001 |
| Current smoking at baseline | 564 (19.9%) | 613 (22.1%) | 810 (23.9%) | 763 (26.4%) | 901 (27.3%) | <0.0001 |
| High total cholesterol at baseline | 784 (27.7%) | 829 (29.9%) | 1,163 (34.2%) | 1,028 (35.6%) | 1,258 (38.1%) | <0.0001 |
| Presence of proteinuria at baseline | 502 (17.7%) | 596 (21.5%) | 740 (21.8%) | 706 (24.5%) | 877 (26.6%) | <0.0001 |
| High baseline serum creatinine | 116 (4.1%) | 91 (3.3%) | 113 (3.3%) | 99 (3.4%) | 129 (3.9%) | 0.94 |
The results in the unadjusted and adjusted Cox regression models comparing each of the 4 upper quintiles to the lowest quintile of baseline HR are listed in Table 3 . In the adjusted Cox models, the uppermost HR quintile was associated with excess event rates of the primary end points, heart failure, sudden cardiac death, myocardial infarction, stroke, and all-cause death. A significant excess of the primary end points, heart failure, myocardial infarction, stroke, and all-cause death was seen also when the second greatest HR quintile was compared to the lowest quintile. These results remained similar after additionally adjusting for the potential confounders.
| Model | Primary Composite End Point | Heart Failure | Sudden Cardiac Death | Myocardial Infarction | Stroke | All-Cause Death |
|---|---|---|---|---|---|---|
| Unadjusted | ||||||
| Highest quintile | 1.69 (1.45–1.97) ⁎ | 2.58 (2.03–3.28) ⁎ | 1.77 (1.28–2.45) ⁎ | 1.35 (1.06–1.71) † | 1.50 (1.14–1.96) † | 1.93 (1.66–2.25) ⁎ |
| Second highest quintile | 1.28 (1.08–1.51) † | 1.48 (1.13–1.94) † | 1.26 (0.88–1.80) | 1.17 (0.91–1.50) | 1.70 (1.30–2.23) ⁎ | 1.40 (1.18–1.65) ⁎ |
| Middle quintile | 1.22 (1.04–1.44) † | 1.60 (1.24–2.07) ⁎ | 1.02 (0.71–1.46) | 1.16 (0.91–1.47) | 1.38 (1.05–1.81) † | 1.14 (0.96–1.35) |
| Second lowest quintile | 1.02 (0.85–1.21) | 1.31 (0.99–1.73) | 1.01 (0.69–1.47) | 0.92 (0.71–1.20) | 1.14 (0.85–1.53) | 1.08 (0.90–1.28) |
| Adjusted for baseline BP ‡ | ||||||
| Highest quintile | 1.77 (1.52–2.07) ⁎ | 2.82 (2.21–3.60) ⁎ | 1.78 (1.28–2.48) ⁎ | 1.40 (1.10–1.77) † | 1.49 (1.14–1.95) † | 2.00 (1.71–2.34) ⁎ |
| Second highest quintile | 1.34 (1.13–1.58) ⁎ | 1.62 (1.24–2.12) ⁎ | 1.28 (0.89–1.83) | 1.20 (0.94–1.55) | 1.73 (1.32–2.26) ⁎ | 1.45 (1.23–1.72) ⁎ |
| Middle quintile | 1.27 (1.08–1.50) † | 1.72 (1.33–2.22) ⁎ | 1.04 (0.72–1.49) | 1.19 (0.93–1.51) | 1.41 (1.07–1.84) † | 1.18 (1.0–1.40) |
| Second lowest quintile | 1.04 (0.87–1.25) | 1.38 (1.04–1.82) † | 1.02 (0.70–1.49) | 0.94 (0.72–1.22) | 1.15 (0.86–1.54) | 1.10 (0.92–1.32) |
| Adjusted for baseline BP ‡ and risk factors § | ||||||
| Highest quintile | 1.73 (1.46–2.04) ⁎ | 2.48 (1.91–3.21) ⁎ | 1.80 (1.27–2.55) ⁎ | 1.48 (1.15–1.90) † | 1.41 (1.06–1.87) † | 1.96 (1.66–2.31) ⁎ |
| Second highest quintile | 1.37 (1.15–1.63) ⁎ | 1.51 (1.14–2.00) † | 1.36 (0.94–1.98) | 1.32 (1.02–1.71) † | 1.70 (1.29–2.25) ⁎ | 1.49 (1.26–1.77) ⁎ |
| Middle quintile | 1.29 (1.09–1.53) † | 1.62 (1.24–2.11) ⁎ | 1.08 (0.75–1.57) | 1.27 (0.99–1.63) | 1.41 (1.07–1.85) † | 1.20 (1.01–1.43) † |
| Second lowest quintile | 1.06 (0.88–1.26) | 1.32 (1.00–1.75) † | 1.06 (0.72–1.55) | 0.98 (0.75–1.28) | 1.18 (0.88–1.58) | 1.13 (0.94–1.35) |
‡ Systolic and diastolic blood pressure.
§ Age, gender, race, body mass index, total cholesterol, smoking, diabetes, proteinuria, history of coronary heart disease, cerebrovascular disease or peripheral arterial disease, left ventricular hypertrophy, and use of β blockers, calcium antagonists, or other antihypertensive drugs.
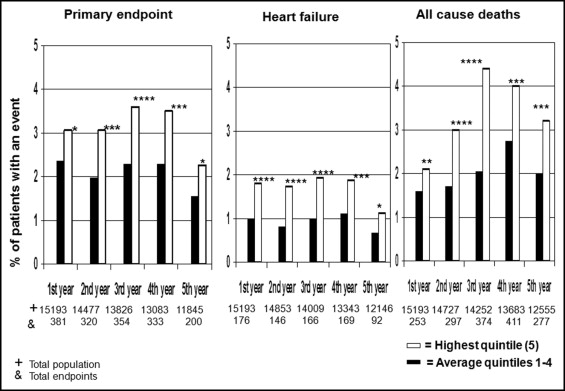
In Figure 1 , we evaluated whether the effect of the baseline HR persisted throughout the trial. Only the incidence of new events occurring separately in each year of the study was considered. The greatest quintile was compared to the average incidence in the pooled 4 lower quintiles of baseline HR. The highest baseline HR quintile was associated with increases in the primary composite end point, heart failure, and total mortality throughout the trial.
The effect of the in-trial HR was evaluated at year 1 of the study for 2 reasons. The first in-trial ECG recording of HR was taken at year 1. At year 1, the BP lowering in the trial reached the nadir and thereafter leveled off. The 13,272 study participants, who did not experience a study end point during the first year, were divided into quintiles of the year 1 HR. The patient characteristics by quintile of in-trial HR are listed in Table 4 . Similar to the trends seen for the baseline HR, the percentage of women, body mass index, baseline BP, diabetes mellitus, and proteinuria tended to be greater in the upper in-trial HR quintiles, but a history of coronary heart disease or taking β blockers was more pronounced in the lowest HR quintiles. In the Cox models adjusted for all covariates, the highest HR quintile was associated with an excess of primary end point, heart failure, sudden death, myocardial infarction, stroke, and total mortality ( Table 5 ). The increase in sudden death was not statistically significant. These results remained similar after additionally adjusting for other confounders. Next, to evaluate whether achieving BP control at year 1 affected the relation between in-trial HR and subsequent cardiovascular events, we divided the population into those with controlled or uncontrolled BP. The average BP was 130/77 ± 7.9/7.1 mm Hg in those with their BP controlled (n = 6,604) and 153/84 ± 13.3/9.3 mm Hg in those with their BP uncontrolled (n = 6,686). The average HR at baseline was 68.7 beats/min in the controlled and 69.3 beats/min in the uncontrolled participants. Figure 2 shows the Kaplan-Meier curves for the primary composite end point in the highest in-trial HR quintile and the pooled 4 lower quintiles in the BP-controlled and BP-uncontrolled groups. A significantly greater incidence of primary end points was seen in the top HR quintile in the BP-controlled group (+53%, p <0.0001, log-rank test) and in the BP-uncontrolled group (+34%, p <0.002). No significant difference was found in the primary events between the upper in-trial HR quintile BP-controlled and BP-uncontrolled groups (p = 0.58). However, among the subjects in the pooled 4 lower quintiles, the group with uncontrolled BP had a significantly greater end point incidence than the group with controlled BP (p = 0.0035).
| Variable | Quintile | p Value | ||||
|---|---|---|---|---|---|---|
| First | Second | Third | Fourth | Fifth | ||
| Subjects (n) | 2,789 | 2,997 | 2,483 | 2,402 | 2,645 | — |
| Age (years) | 67.0 ± 7.7 | 67.0 ± 8.0 | 67.2 ± 8.1 | 66.9 ± 8.2 | 67.1 ± 8.4 | 0.83 |
| HR at year 1 (beats/min) | 54.2 ± 4.1 | 63.1 ± 2.0 | 69.4 ± 1.7 | 76.3 ± 2.3 | 89.9 ± 8.6 | — |
| Year-1 HR quintile interval (beats/min) | 40.0–59.0 | 60.0–66.0 | 67.0–72.0 | 73.0–80.0 | 81.0–137.0 | — |
| Women | 895 (32.1%) | 1,174 (39.2%) | 1,112 (44.8%) | 1,161 (48.3%) | 1,272 (48.1%) | <0.0001 |
| White | 2,525 (90.5%) | 2,708 (90.4%) | 2,225 (89.6%) | 2,139 (89.1%) | 2,342 (88.5%) | 0.004 |
| Body mass index (kg/m 2 ) | 28.2 ± 4.3 | 28.3 ± 4.9 | 28.6 ± 4.9 | 28.8 ± 5.1 | 29.3 ± 5.7 | <0.0001 |
| Baseline diastolic blood pressure (mm Hg) | 86.3 ± 10.9 | 87.2 ± 10.8 | 87.5 ± 10.8 | 88.1 ± 10.4 | 88.8 ± 10.5 | <0.0001 |
| Baseline systolic blood pressure (mm Hg) | 154 ± 19.4 | 155 ± 19.2 | 154 ± 18.9 | 155 ± 18.3 | 156 ± 19.0 | <0.0001 |
| In-trial diastolic blood pressure (mm Hg) | 79.7 ± 7.2 | 80.2 ± 6.8 | 80.3 ± 6.7 | 80.8 ± 6.6 | 81.4 ± 6.7 | <0.0001 |
| In-trial systolic blood pressure (mm Hg) | 140 ± 11.8 | 140 ± 11.1 | 139 ± 10.6 | 140 ± 10.9 | 141 ± 11.5 | <0.0001 |
| Antihypertensives at randomization | 2,635 (94.6%) | 2,804 (93.6%) | 2,300 (92.8%) | 2,215 (92.3%) | 2,424 (91.8%) | <0.0001 |
| β Blockers | 1,231 (44.2%) | 1,081 (36.1%) | 806 (32.5%) | 652 (27.2%) | 697 (26.4%) | <0.0001 |
| Coronary heart disease | 1,548 (55.5%) | 1,451 (48.4%) | 1,084 (43.7%) | 975 (40.6%) | 1,017 (38.4%) | <0.0001 |
| Peripheral arterial disease | 341 (12.2%) | 426 (14.2%) | 342 (13.8%) | 357 (14.9%) | 377 (14.3%) | 0.025 |
| Stroke or transient ischemic attack | 569 (20.4%) | 600 (20.0%) | 496 (20.0%) | 463 (19.3%) | 520 (19.7%) | 0.36 |
| Left ventricular hypertrophy with strain pattern | 180 (6.5%) | 177 (5.9%) | 132 (5.3%) | 121 (5.0%) | 148 (5.6%) | 0.062 |
| Diabetes mellitus | 573 (20.5%) | 801 (26.7%) | 723 (29.1%) | 829 (34.5%) | 1,164 (44.0%) | <0.0001 |
| Current smoking at baseline | 545 (19.5%) | 668 (22.3%) | 584 (23.5%) | 652 (27.1%) | 742 (28.1%) | <0.0001 |
| High baseline total cholesterol | 790 (28.3%) | 972 (32.4%) | 833 (33.5%) | 906 (37.7%) | 980 (37.1%) | <0.0001 |
| Presence of proteinuria at baseline | 553 (19.8%) | 602 (20.1%) | 530 (21.4%) | 552 (23.1%) | 712 (26.9%) | <0.0001 |
| High baseline serum creatinine | 106 (3.8%) | 87 (2.9%) | 76 (3.1%) | 74 (3.1%) | 104 (3.9%) | 0.73 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


