Myocardial fractional flow reserve (FFR) has emerging clinical utility and prognostic value in medically stabilized patients with non–ST-segment elevation myocardial infarction (NSTEMI). The aim of this study was to investigate whether measurement of FFR compared to coronary angiography alone improves diagnostic efficiency in patients with NSTEMIs. One hundred consecutive patients with NSTEMIs who had previously undergone clinically indicated FFR measurements were included. In a simulated decision exercise, 5 interventional cardiologists retrospectively and independently reviewed the clinical history and coronary angiogram of each patient and then made a treatment decision. FFR results were then disclosed, and the same cardiologists were asked to review their initial treatment decisions. A p value <0.05 indicates a difference between cardiologists. The proportion of patients allocated to each treatment option initially differed among the 5 cardiologists (p = 0.0061). Forty-two percent of all FFR measurements were made in culprit lesions. After FFR disclosure, the number of patients in whom the treatment decisions made by each cardiologist independently conformed (and so represented the majority with ≥3 of the 5 cardiologists) increased from 65% to 91% (p = 0.0094). After FFR disclosure, the cardiologists changed their initial treatment plans in 46% of patients (p = 0.0016). Changes in favor of medical therapy occurred in 24% of patients (p = 0.0016), and this increase was associated with reductions in “deferred” management (p = 0.0067), single-vessel percutaneous coronary intervention (p = 0.0052), and multivessel percutaneous coronary intervention (p = 0.046). In conclusion, FFR measurement reduced diagnostic variability and changed cardiologists’ treatment decisions for patients with NSTEMIs.
Although the diagnostic and clinical utility of fractional flow reserve (FFR) measurement in non–ST-segment elevation myocardial infarction (NSTEMI) has emerging importance, FFR adoption is low. Therefore, we set out to investigate some of the uncertainties in this area and more specifically whether cardiologists might actually be influenced by FFR results when made available in unselected patients with NSTEMIs. Our first aim was to determine whether FFR measurement would alter treatment decisions in invasively managed patients with NSTEMIs. Our second aim was to determine whether FFR disclosure would improve diagnostic certainty. Our third aim was to determine the factors that might influence the degree of compliance of cardiologists with FFR results.
Methods
We simulated the catheterization laboratory management of a cohort of actual patients with NSTEMIs in whom pressure-wire studies had been performed because of diagnostic uncertainty on the basis of visual assessment of the coronary angiogram. The study was approved by the Clinical Governance Department of Golden Jubilee National Hospital.
Consecutive patients with NSTEMIs who had undergone clinically indicated coronary angiography with FFR measurement from January 2009 to March 2010 were included. Golden Jubilee National Hospital is a regional cardiothoracic center with 12 interventional cardiologists. In our hospital, treatment decisions for medical therapy, percutaneous coronary intervention (PCI; including bare-metal and drug-eluting stents), coronary artery bypass grafting (CABG), and deferred management in patients with NSTEMI are made according to contemporary guidelines.
Patients were identified from the hospital’s catheterization laboratory registry, which prospectively accumulates the clinical data of all patients who undergo invasive management. The inclusion criteria were (1) a diagnosis of recent (≤5 days) NSTEMI and (2) an FFR measurement intended to guide diagnostic management during coronary angiography. The only exclusion criterion was an FFR measurement for other reasons (e.g., FFR measured after stent deployment). The definition of a culprit lesion was a stenosis in a coronary artery causally implicated in the NSTEMI, on the basis of angiographic appearances (e.g., eccentric, hazy appearance) and regional ischemic electrocardiographic changes or wall motion abnormalities on echocardiography. The definition of a nonculprit stenosis was a lesion >30% of the reference vessel diameter not implicated in the acute presentation.
The indication for FFR measurement was the presence of an intermediate coronary lesion (i.e., 40% to 80% stenosis severity) associated with diagnostic and treatment uncertainty. FFR measurement was used to provide functional information on lesion severity, and FFR ≤0.80 was taken to represent a flow-limiting stenosis. An FFR threshold of 0.80 identifies ischemia-causing coronary stenoses with high diagnostic accuracy (>90% sensitivity and specificity).
FFR measurements were obtained according to standard practice in our hospital using a 0.014-inch pressure-sensitive wire (RADI; St. Jude Medical Corporation, Uppsala, Sweden) during intravenous adenosine infusion (140 μg/kg/min) to establish maximal coronary hyperemia and confirmed with typical changes in blood pressure and symptoms.
The study simulated the actual clinical decision-making process by taking place in the cardiac catheterization laboratory (September to November 2010). Five accredited interventional cardiologists (A, B, C, D, and E) independently reviewed each case of NSTEMI, and a sixth cardiologist acted as the study coordinator.
These cardiologists had a broad range of postaccreditation experience: 1 year (cardiologist A), 2 years (cardiologist B), 5 years (cardiologist C), 15 years (cardiologist E), and 30 years (cardiologist D). Three of the cardiologists had worked in the United States, and 4 of the cardiologists had worked in Canada. Each cardiologist performed ≥250 PCIs per year.
Each interventional cardiologist met with the coordinator separately. The cardiologists were blinded to patient identity and the original treatment decisions ( Table 1 ). Each patient’s clinical records and coronary angiogram were reviewed, and on the basis of this information, the cardiologist then made a management decision. The FFR results were then disclosed, and the initial management decision was reviewed in light of the FFR result and changed (or not), as appropriate.
| Characteristic | Value |
|---|---|
| Age (yrs), mean ± SD | 64 ± 11 |
| Men | 75 |
| Diabetes mellitus | 21 |
| Previous myocardial infarction | 34 |
| Previous stroke | 5 |
| Previous CABG | 7 |
| Previous PCI | 16 |
| Time from presentation to angiography (days), median (interquartile range) | 2.5 (1.3–4.0) |
| Number of coronary arteries narrowed | |
| 1 | 26 |
| 2 | 29 |
| 3 | 45 |
| Lesion type in each patient | |
| Culprit lesion | 42 |
| Nonculprit lesion | 38 |
| Culprit and nonculprit | 8 |
| Uncertain clinical significance | 12 |
| Single lesion | 94 |
| Tandem lesions | 6 |
| Actual treatment | |
| Medical therapy | 34 |
| Single-vessel PCI | 40 |
| Multivessel PCI | 13 |
| CABG | 13 |
The initial treatment decisions of the 5 cardiologists (A, B, C, D, and E) on the basis of visual assessment of the angiogram alone were assessed using a chi-square test. Comparisons among the 5 cardiologists for each treatment option before and after disclosure of the FFR results were assessed using Fisher’s exact test. Consensus (or majority) treatment decisions were defined as ≥3 of the 5 cardiologists’ agreeing on the same treatment decision.
For our third aim, we studied the patient and operator characteristics that might influence compliance with an FFR result and propensity to change an initial treatment decision. We created a proportional odds model on 3 possible outcomes: (1) no change, (2) change to medical management, and (3) change to revascularization with either PCI or CABG with rater (i.e., the cardiologist) as a random effect and an independent covariance matrix. This approach yielded univariate odds models for the following characteristics: each cardiologist (rater), gender, clinical significance of the lesion (culprit, nonculprit, or not determinable), diabetes, previous CABG, previous myocardial infarction, previous PCI, and age. All of these models were adjusted for rater. A multivariate odds model was created adjusting simultaneously for all these characteristics, regardless of statistical significance. The odds ratio is the cumulative odds ratio (outcome 2 vs outcome 1, i.e., change to medical management vs no change, and then outcome 3 vs outcomes 1 and 2, i.e., change to revascularization with either PCI or CABG vs change to medical management or no change). An odds ratio >1 indicates a higher probability of a subject with that characteristic making the specified change.
All p values <0.05 were taken as significant, and no adjustment was made for multiple comparisons. Statistical analysis was performed using SAS version 9.2 (SAS Institute Inc., Cary, North Carolina).
Results
FFR was measured in 109 patients with NSTEMIs (January 1, 2009, to March 31, 2010). Nine of these patients were excluded because FFR was measured after stenting (n = 8) or because FFR measurement failed because of vessel tortuosity (n = 1). Therefore, 100 different patients with NSTEMIs were included in the study, and their clinical characteristics and actual treatments are listed in Table 1 . Of all PCI procedures (n = 66), 33 did not involve FFR. Representative cases are shown in Figures 1 and 2 .
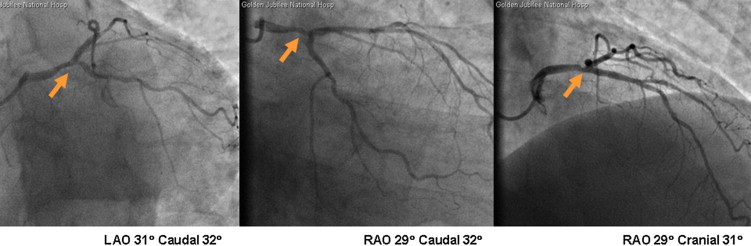
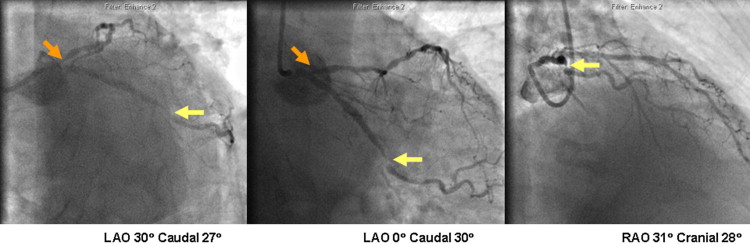
The initial treatment decisions on the basis of clinical data alone differed overall among the 5 cardiologists (p = 0.0061).
Changes in treatment decisions after FFR disclosure are listed in Tables 2 to 4 . The number of patients in whom the treatment decisions made by each cardiologist independently conformed (and so represented a majority with ≥3 of the 5 cardiologists) increased from 65% on the basis of coronary angiography alone to 91% after FFR disclosure (p = 0.0094; Table 4 ).
| Treatment Decision | Cardiologist (Rater) | p Value | Overall | ||||
|---|---|---|---|---|---|---|---|
| A | B | C | D | E | |||
| Changed to medical management | 30 | 23 | 29 | 22 | 16 | 0.12 | 24.0% |
| Changed to PCI/CABG | 8 | 7 | 8 | 11 | 8 | 0.91 | 8.4% |
| No change | 62 | 70 | 63 | 67 | 76 | 0.20 | 67.6% |
| Type of Change | Direction of Change | Cardiologist (Rater) | Average Change | Rater Effect p Value | ||||
|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | ||||
| No change | 48% | 51% | 53% | 51% | 69% | 54% | ||
| Any change | 52% | 49% | 47% | 49% | 31% | 46% | 0.0016 | |
| Medical management | To | 30% | 23% | 29% | 22% | 16% | 24% | 0.0064 |
| From | 4% | 4% | 0% | 2% | 3% | 3% | NA | |
| Single vessel PCI | To | 16% | 18% | 13% | 18% | 14% | 16% | 0.68 |
| From | 22% | 21% | 14% | 14% | 8% | 16% | 0.0052 | |
| Multivessel PCI | To | 5% | 5% | 3% | 5% | 1% | 4% | NA |
| From | 10% | 12% | 8% | 10% | 4% | 9% | 0.046 | |
| CABG | To | 1% | 3% | 2% | 4% | 0% | 2% | NA |
| From | 0% | 4% | 1% | 0% | 3% | 2% | NA | |
| Deferred management | To | 0% | 0% | 0% | 0% | 0% | 0.0% | NA |
| From | 16% | 8% | 24% | 23% | 12% | 17% | 0.0067 | |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


