Fetuin-A is a ubiquitous anti-inflammatory glycoprotein that counteracts proinflammatory cytokine production. Previous studies have shown that low fetuin-A concentration is associated with cardiovascular death and may play an important role in the prognosis of patients with acute coronary syndromes (ACS). The purpose of this study was to assess in large cohort of patients admitted for ACS the prognostic value of fetuin-A adjusted for C-reactive protein value (CRP) and Global Registry of Acute Coronary Events (GRACE) risk score. Plasma fetuin-A and CRP concentrations were measured on day 3 in 754 consecutive patients with ACS (mean age 66 ± 14 years, 404 with ST-segment elevation and 350 without ST-segment elevation) included in the French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction (FAST-MI), and these data were correlated to 1-year mortality. Plasma fetuin-A and CRP concentrations at admission averaged 95 ± 27 and 12 ± 16 mg/L, respectively. Overall, 1-year cardiovascular mortality was 10% (28 in-hospital deaths and 51 deaths after discharge), 17% in patients with low fetuin-A (less than the first tertile), 18% with high CRP (higher than the third tertile), and 23% in patients with low fetuin associated with high CRP (p <0.01). In contrast, patients with neither low fetuin-A nor high CRP had a low mortality rate (5%). Multivariate analysis adjusted for GRACE risk score showed that low fetuin-A and high CRP concentration remained associated with outcomes (odds ratio 2.28, 95% confidence interval 1.20 to 4.33). In conclusion, fetuin-A combined with CRP level is associated with cardiovascular death in patients with ACS.
After an acute myocardial infarction, proinflammatory cytokines are secreted and anti-inflammatory cytokines are reduced to maintain the healing process. The balance between proinflammatory and anti-inflammatory pathways is a determinant of outcome, and an inadequate inflammatory balance leading to excessive production of proinflammatory cytokines is deleterious for left ventricular (LV) remodeling and paves the way for the development of heart failure. Fetuin-A/α2 Heremans-Schmid glycoprotein is an anti-inflammatory mediator produced by the liver and is involved in macrophage deactivation. Fetuin-A enhances the cellular uptake of cationic inhibitors of proinflammatory cytokine synthesis and thus prevents the self-amplification of the inflammatory response. Low fetuin-A concentration is associated with more severe cardiovascular outcomes after myocardial infarction with ST-segment elevation or in patients with end-stage renal disease. Several investigators have suggested that fetuin-A exerts a protective effect against ischemia in cardiomyocytes, and a recent experimental study demonstrated that fetuin-A infusion may be used as a therapy to reduce ischemia-related lesions in a stroke model. In contrast, controversial studies have demonstrated that high fetuin-A is associated with metabolic syndrome and may increase the risk for cardiovascular diseases, especially in patients with diabetes, by stimulating adipocyte-related inflammatory cytokine production. Regarding these controversial data, in this study the prognostic value of low fetuin-A in acute coronary syndromes (ACS) was evaluated in a large population with adjustment for C-reactive protein (CRP) and clinical markers.
Methods
The methods of the French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction (FAST-MI) have been described previously in detail. The objective of FAST-MI was to gather complete and representative data on the management and outcomes of patients admitted to intensive care units for definite acute myocardial infarction, irrespective of the types of institutions to which they were admitted (i.e., university hospitals, public hospitals, or private clinics). All consecutive adult patients (aged ≥18 years) were included in the registry if they had elevated serum markers of myocardial necrosis >2 times the upper limit of normal for creatine kinase, creatine kinase-MB, or elevated troponins, and either symptoms compatible with acute myocardial infarction and/or electrocardiographic changes in ≥2 contiguous leads with pathologic Q waves (≥0.04 seconds) and/or persisting ST-segment elevation or depression >0.1 mV. The time from symptom onset to intensive care unit admission had to be <48 hours. Patients were managed according to usual practice; treatment was not affected by participation in the registry. On day 3 after the onset of ACS, blood samples were taken to create a serum bank. The duration of recruitment was 31 days per center for all patients and 2 months for patients with diabetes, ranging from October 1 to December 24, 2005. Of the 374 centers in France that treated patients with acute myocardial infarction at that time, 223 (60%) participated in the registry. Among these, 100 centers recruited 754 patients who contributed to the serum fetuin-A analysis. Written informed consent was provided by each patient. The study was reviewed by the Committee for the Protection of Human Subjects in Biomedical Research of Saint Antoine University Hospital, and the data file was declared to Commission Nationale de l’Informatique et des Libertés.
Follow-up data were collected through direct contact with the patients’ physicians, the patients or their families, and the registry offices of their birthplaces. Follow-up was completed in all patients. The primary outcome was defined as death at 1 year, and the secondary outcome was long-term survival at 3 years.
Blood samples were taken on day 3 and stored at −80°C at the Department of Clinical Pharmacology, University of Pierre and Marie Curie. All samples were anonymized and analyzed in random order. We measured serum fetuin-A concentrations using a semiautomated sandwich immune-enzymometric assay (EDI Human Fetuin-A ELISA, Epitope Diagnostics, San Diego, California). Briefly, a purified human fetuin-A-specific polyclonal antibody was used as the capture antibody, and another purified fetuin-A-specific antibody from a different animal species (labeled) was used as the detection antibody. The first incubation time, substrate incubation time, and antibody tracer incubation time were 2 hours, 30 minutes, and 20 minutes, respectively. Reagent distribution and predilution of samples were performed using an automated workstation (Freedom Evo; Tecan, Mannedort, Switzerland). The final dilution reached after a cascade of 3 successive dilutions was 1/4,096. The lowest level of detection for human fetuin-A was 0.025 g/L and was calculated as the mean + 3 SDs of 10 successive measurements of assay buffer samples. The linear measurement ranged up to 7 g/L according to the manufacturer’s specifications. Variability was assessed on 4 different series, and the result was close to the manufacturer’s specifications. Intra-assay variation was <5.5% and interassay variation <6.8%, determined on 9 different samples tested, with concentrations ranging from 0.110 to 0.260 g/L. We measured CRP concentrations by immune-nephelometry (Dade Behring, Paris, France) in all patients. The lowest level of detection and total imprecision were 0.5 mg/L and 4.6% at 4 mg/L, respectively, and 0.1 mg/L and <5% at 0.5 mg/L, respectively.
The sample size was set with the α risk and statistical power (1–β) defined at 5% and 80%, respectively. According to 1-year mortality previously reported in ACS (10%) and on the basis of the hypothesis that mortality risk would be increased by 33% in patients with low fetuin-A, the sample size should be ≥702. Tertiles were used to define low (first tertile) and high (third tertile) concentrations of CRP and fetuin-A. Severe inflammatory imbalance was defined by a low fetuin-A concentration associated with a high CRP concentration. Baseline clinical characteristics and outcomes of patients with severe inflammatory imbalances were compared to those of patients with neither low fetuin-A nor high CRP concentrations. We used a multivariate Cox proportional-hazards model to assess the independent prognostic value of variables with the outcome events during the follow-up period. The proportional-hazards assumption was checked graphically by plotting scaled Schoenfeld residuals against time with the LOESS smoothing function used to test for nonproportionality. Stepwise multivariate analysis was used to define independent predictors of death, and covariates included in the model were Global Registry of Acute Coronary Events (GRACE) risk score, history of heart failure, history of diabetes, the LV ejection fraction, in-hospital medical treatment, revascularization therapy, fetuin-A, and CRP concentration. Results are expressed as hazard ratios for Cox models with 95% confidence intervals. The incremental value of risk factors for predicting mortality was assessed by comparing the global chi-square statistic of the logistic model. All statistical tests, performed using SAS version 9.1 (SAS Institute Inc., Cary, North Carolina), were 2 sided, and p values <0.05 were considered significant.
Results
The study included 754 patients (mean age 66 ± 14 years, 73% men) admitted for ACS (54% with ST-segment elevation myocardial infarctions). Baseline population characteristics are listed in Table 1 . Most patients (70%) were treated with percutaneous coronary intervention and received aspirin (93%), clopidogrel (92%), and β blockers (74%) at admission. However, only 56% of patients received angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists, and the association of β blockers, aspirin, statins, and angiotensin-converting enzyme inhibitors or angiotensin receptor antagonists was only fully delivered to <50% of the population (41% [315 of 754]). The mean GRACE score was 144 ± 35, and 23% had symptomatic heart failure. In-hospital death occurred in 28 patients, and after discharge, 51 additional patients died at 1 year. During the first year, recurrent ACS were observed in 21 patients (2.7%), and stroke occurred in 6 patients. Overall in-hospital and 1- and 3-year mortality rates were 4% (11 of 754), 11% (79 of 754), and 18% (132 of 754), respectively.
| Variable | Fetuin-A Tertile | CRP Tertile | ||||||
|---|---|---|---|---|---|---|---|---|
| First (n = 250) | Second (n = 250) | Third (n = 254) | p Value | First (n = 257) | Second (n = 246) | Third (n = 251) | p Value | |
| Age (yrs) | 70 ± 12 | 64 ± 14 | 63 ± 13 | <0.0001 | 63 ± 13 | 65 ± 14 | 69 ± 13 | <0.0001 |
| Body mass index (kg/m 2 ) | 26 ± 5 | 27 ± 5 | 28 ± 4 | 0.006 | 27 ± 4 | 27 ± 4 | 27 ± 5 | 0.5 |
| Hypertension | 63% | 54% | 61% | 0.10 | 52% | 60% | 66% | 0.007 |
| Diabetes mellitus | 35% | 26% | 31% | 0.10 | 24% | 28% | 41% | <0.001 |
| Previous ischemic stroke | 8% | 2% | 4% | 0.005 | 3% | 4% | 8% | 0.02 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) ∗ | 73 ± 37 | 77 ± 27 | 76 ± 25 | 0.25 | 79 ± 25 | 76 ± 35 | 71 ± 29 | 0.006 |
| CRP (mg/L) | 16 ± 19 | 12 ± 8 | 8 ± 10 | <0.0001 | 1 ± 1 | 6 ± 3 | 28 ± 20 | <0.0001 |
| Fetuin-A (mg/L) | 65 ± 19 | 95 ± 6 | 122 ± 15 | <0.0001 | 100 ± 28 | 96 ± 27 | 86 ± 25 | <0.0001 |
| Non–ST-segment elevation myocardial infarction | 48% | 42% | 49% | 0.20 | 46% | 43% | 51% | 0.20 |
| Percutaneous coronary intervention | 66% | 74% | 71% | 0.09 | 74% | 72% | 65% | 0.06 |
| Optimal medical treatment † | 39% | 40% | 46% | 0.30 | 43% | 46% | 37% | 0.07 |
| GRACE risk score | 156 ± 38 | 140 ± 32 | 138 ± 32 | <0.0001 | 135 ± 30 | 143 ± 34 | 157 ± 37 | <0.0001 |
| In-hospital events | ||||||||
| LV ejection fraction <40% | 25% | 16% | 17% | 0.04 | 8% | 19% | 32% | <0.0001 |
| In hospital mortality | 6% | 3% | 2% | 0.05 | 3% | 3% | 6% | 0.20 |
| 1-yr outcomes | ||||||||
| 1-yr mortality | 17% | 8% | 6% | <0.0001 | 6% | 8% | 18% | <0.0001 |
| 1-yr recurrent ACS | 2% | 3% | 4% | 0.60 | 2% | 3% | 3% | 0.80 |
| 1-yr recurrent stroke | 1% | 0% | 1% | 0.20 | 1% | <1% | 1% | 0.60 |
| 3-yr outcomes | ||||||||
| Death | 24% | 15% | 13% | 0.002 | 9% | 14% | 29% | <0.001 |
| Recurrent stroke | 2% | 1% | 2% | 0.80 | 3% | <1% | 1% | 0.04 |
| Recurrent ACS | 3% | 4% | 4% | 0.40 | 4% | 4% | 3% | 0.70 |
∗ By Modification of Diet in Renal Disease equation.
† Nonoptimal treatment was defined when 1 of the following treatments was missing at discharge: angiotensin-converting enzyme inhibitor or angiotensin receptor antagonist, β blocker, aspirin, and statin.
The mean CRP concentration was 12 ± 16 mg/L (range 0 to 111; first tertile <2.5 mg/L, n = 257; third tertile >13 mg/L, n = 251). Characteristics of patients according to CRP levels are listed in Table 1 . The 1-year mortality rate according to CRP tertile was 6% (14 of 243) for the first tertile, 8% (20 of 246) for the second tertile, and 18% (45 of 251) for the third tertile. Using receiver-operating characteristic curve analysis (area under the curve 0.64, p <0.001), the optimal cut-off value of CRP identified death with sensitivity and specificity of 71% and 53%, respectively. Interestingly, CRP levels were not associated with 1- and 3-year recurrent stroke and ACS.
Overall, fetuin-A concentration averaged 95 ± 27 mg/L (range 5 to 190; first tertile <83 mg/L, n = 250; third tertile >105 mg/L, n = 254). Characteristics of patients according to fetuin-A tertile are listed in Table 1 . Fetuin-A concentration was correlated with CRP concentration (r = −0.20, p <0.001; Table 1 ), body mass index, and age (r = −0.18, p <0.001) and was lower in patients with history of stroke and in the 48 patients with body mass index <21 kg/m 2 (82 ± 30 vs 95 ± 27 mg/L, p = 0.0009). No association was observed with cholesterol level, diabetes (94 ± 27 vs 94 ± 27 mg/L, p = 0.60), or white blood cell concentration. Low fetuin-A level was associated with a higher GRACE score and more frequent LV dysfunction and in-hospital mortality ( Table 1 ). One-year mortality according to fetuin-A tertile was 17% (43 of 207) for the first tertile (<83 mg/L), 8% (20 of 230) for the second tertile, and 6% (16 of 238) for the third tertile (p <0.0001). According to receiver-operating characteristic curve analysis (area under the curve 0.63, p <0.001), the optimal cut-off value for fetuin-A (<91 mg/L) identified death with sensitivity and specificity of 61% and 67%, respectively. Similarly to CRP levels, fetuin-A levels were not associated with 1- and 3-year recurrent stroke and ACS.
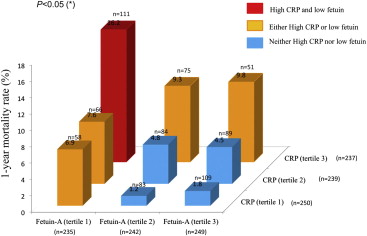
When combining fetuin-A and CRP value (p for interaction <0.05), the mortality rate was 5% (18 of 372) in patients with neither low fetuin-A nor high CRP (n = 372), 13% in patients with either high CRP or low fetuin-A (34 of 263), and 23% in those with high CRP and low fetuin-A (27 of 119) ( Figure 1 ). Compared to CRP and fetuin-A alone, the ratio of CRP to fetuin-A was better associated with death (area under the curve 0.66, p <0.0001). The optimal cut-off value of CRP and fetuin-A (>0.06) identified death with 71% sensitivity and 66% specificity. Despite a significant association with death, the combined value of fetuin-A and CRP failed to correlate with recurrent ACS and stroke ( Table 2 ). Patients with low fetuin-A and high CRP concentrations were older and had more frequent histories of diabetes mellitus, coronary artery bypass graft surgery, and ischemic stroke compared to patients with neither low fetuin-A nor high CRP concentration or either low fetuin-A or high CRP concentration. In addition, percutaneous coronary intervention was less frequently attempted, and heart failure symptoms, LV dysfunction (LV ejection fraction <40%), and GRACE risk score were more severe in patients with low fetuin-A and high CRP concentrations compared to the other groups. Multivariate analysis including these covariates demonstrated that low fetuin-A combined with high CRP (CRP and fetuin-A ratio or CRP and fetuin-A tertiles; Table 3 ) was associated with the risk for death at 1 year (odds ratio 2.28, 95% confidence interval 1.20 to 4.33, p = 0.01; Table 3 ). Figure 2 shows that fetuin-A combined with CRP had a significant incremental value over GRACE risk score, history of heart failure, diabetes, and in-hospital treatment for predicting death. Figures 3 and 4 represent the Kaplan-Meier survival curves at 1 and 3 years according to fetuin-A and CRP concentrations. At 3 years, death occurred in 132 patients (17.9%): in 34% of patients with low fetuin-A and high CRP, in 20% of patients with either low fetuin-A or high CRP, and in 11% (p <0.0001) of patients with neither low fetuin-A nor high CRP. Low fetuin-A and high CRP concentration remained associated with mortality by multivariate analysis (odds ratio 1.78, 95% confidence interval 1.10–2.88, p = 0.02; Figure 4 ).
| Variable | All Patients | First Tertile of Fetuin-A/Third Tertile of CRP | Either First Tertile of Fetuin-A or Third Tertile of CRP | Neither First Tertile of Fetuin nor Third Tertile of CRP | p Value |
|---|---|---|---|---|---|
| (n = 754) | (n = 119) | (n = 263) | (n = 372) | ||
| Age (yrs) | 66 ± 14 | 71 ± 13 | 67 ± 13 | 62 ± 13 | <0.0001 |
| Previous coronary artery bypass grafting | 36 (5%) | 13 (11%) | 8 (3%) | 15 (4%) | 0.005 |
| Body mass index (kg/m 2 ) | 27 ± 5 | 26 ± 5 | 27 ± 5 | 27 ± 4 | 0.10 |
| Previous ischemic stroke | 35 (5%) | 12 (10%) | 15 (6%) | 8 (2%) | <0.0001 |
| Diabetes mellitus | 234 (31%) | 49 (41%) | 94 (36%) | 91 (24%) | <0.001 |
| Previous lipid-lowering treatment | 400 (53%) | 63 (53%) | 150 (57%) | 187 (50%) | 0.30 |
| Non–ST-segment elevation myocardial infarction | 350 (46%) | 65 (54%) | 118 (45%) | 167 (45%) | 0.15 |
| GRACE risk score | 144 ± 35 | 163 ± 39 | 150 ± 35 | 134 ± 30 | <0.0001 |
| Treatment | |||||
| Aspirin | 699 (93%) | 107 (90%) | 242 (92%) | 350 (94%) | 0.43 |
| Clopidogrel | 692 (92%) | 107 (90%) | 239 (91%) | 346 (93%) | 0.60 |
| Glycoprotein IIb/IIIa inhibitors | 342 (45%) | 48 (40%) | 124 (47%) | 171 (46%) | 0.43 |
| Angiotensin receptor blockers | 423 (56%) | 68 (57%) | 147 (56%) | 208 (56%) | 0.98 |
| β blockers | 557 (74%) | 77 (65%) | 189 (72%) | 290 (78%) | 0.06 |
| Insulin | 86 (11%) | 26 (22%) | 33 (12.7%) | 27 (7.2%) | <0.0001 |
| Percutaneous coronary intervention | 531 (70%) | 69 (58%) | 189 (72%) | 273 (74%) | 0.005 |
| Optimal medical treatment ∗ | 315 (42%) | 35 (29%) | 119 (45%) | 161 (43%) | 0.01 |
| Biologic data | |||||
| Glycemia at day 1(g/L) | 1.5 ± 0.7 | 1.7 ± 1.0 | 1.6 ± 0.8 | 1.4 ± 0.5 | <0.0001 |
| Estimated glomerular filtration rate (ml/min/1.73 m 2 ) † | 75 ± 30 | 70 ± 27 | 73 ± 38 | 79 ± 24 | 0.01 |
| Troponin I (UI) | 42 ± 98 | 40 ± 88 | 52 ± 126 | 36 ± 78 | 0.21 |
| In-hospital events | |||||
| Congestive heart failure (Killip class ≥II) | 178 (23%) | 44 (37%) | 82 (31%) | 52 (14%) | <0.0001 |
| LV ejection fraction <40% | 113 (15%) | 33 (28%) | 44 (17%) | 36 (10%) | <0.0001 |
| Death | 28 (4%) | 8 (7%) | 13 (5%) | 7 (2%) | 0.02 |
| 1-yr outcomes | |||||
| Death | 79 (11%) | 27 (23%) | 34 (13%) | 18 (5%) | <0.0001 |
| Recurrent stroke | 6 (<1%) | 1 (<1%) | 3 (1.1%) | 2 (<1%) | 0.70 |
| Recurrent ACS | 21 (3%) | 3 (3%) | 6 (2%) | 12 (3%) | 0.70 |
| 3-yr outcomes | |||||
| Death | 132 (18%) | 41 (34%) | 52 (20%) | 39 (11%) | <0.001 |
| Recurrent stroke | 12 (2%) | 2 (2%) | 5 (2%) | 5 (1%) | 0.80 |
| Recurrent ACS | 28 (4%) | 3 (3%) | 10 (4%) | 15 (4%) | 0.70 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


