The current guidelines most strongly support cardiac resynchronization therapy (CRT) for patients with heart failure with a QRS width of ≥150 ms and left bundle branch block (LBBB). Our objective was to assess the potential benefit of echocardiographically guided left ventricular (LV) lead positioning for patients with a QRS width <150 ms or non-LBBB as a substudy of the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region (STARTER) prospective, randomized controlled trial. The STARTER trial randomized 187 patients with heart failure, a QRS of ≥120 ms, and ejection fraction of ≤35% to LV lead guided to the site of latest mechanical activation by speckle tracking radial strain versus routine implantation. The predefined primary end point was heart failure hospitalization or death within 2 years. This substudy included 151 CRT patients with matching echocardiographic and LV lead position data and complete follow-up data. Patients with a QRS width of 120 to 149 ms or non-LBBB and LV lead concordant or adjacent to the site of latest mechanical activation had favorable outcomes after CRT similar to those with LBBB or a QRS width of ≥150 ms. In contrast, patients with a QRS of 120 to 149 ms or non-LBBB and remote LV leads had unfavorable outcomes (hazard ratio 5.45, 95% confidence interval 2.36 to 12.6, p <0.001, and hazard ratio 4.92, 95% confidence interval 2.12 to 11.39, p <0.001, respectively, with significant interaction after adjusting for baseline variables, p = 0.038 and p = 0.008). In conclusion, LV lead positioning with respect to the echocardiographic site of latest activation was significantly associated with more favorable clinical outcomes in patients with a QRS duration <150 ms and/or non-LBBB. Additional prospective study is warranted.
Cardiac resynchronization therapy (CRT) has significantly improved the outcome of many patients with heart failure (HF) with QRS widening and depressed left ventricular (LV) function. Several large randomized clinical trials demonstrating the benefit of CRT used the selection criterion of a QRS width of ≥120 or 130 ms. The recent guidelines most strongly support CRT for patients with a QRS width of ≥150 ms and left bundle branch block (LBBB) morphology, based on their most consistent response to CRT. However, >1/3 of potential CRT candidates with a widened QRS have a QRS duration of 120 to 150 ms and/or non-LBBB, and it is unclear how to optimize patient selection or CRT delivery for these patients. Two recent randomized clinical trials, the Speckle Tracking Assisted Resynchronization Therapy for Electrode Region (STARTER) trial and the Targeted Left Ventricular Lead Placement to Guide Cardiac Resynchronization Therapy (TARGET) trial, demonstrated the clinical benefits of echocardiographic speckle tracking radial strain-guided lead placement toward the site of latest activation compared with a routine approach. The objective of the present substudy of the STARTER trial was to assess the effect of echocardiographically guided lead positioning on CRT for patients classified using the recent guidelines and grouped by QRS width 120 to 150 ms versus those with a QRS width of ≥150 ms and grouped by non-LBBB morphology versus LBBB morphology.
Methods
The present study was a substudy of the prospective, controlled, double-blind, randomized STARTER trial, which had enrolled and randomized 187 patients to either routine or echocardiographically guided LV lead implantation in a 2:3 fashion. The institutional review board approved the present study, and all patients gave written informed consent. The inclusion and exclusion criteria have been previously published. In brief, patients aged >18 years, with New York Heart Association class II-IV chronic HF of both ischemic and nonischemic etiologies, with an LV ejection fraction of ≤35% and a QRS duration of ≥120 ms were included. Patients with right ventricular pacing were included in the present study, as were patients with persistent atrial arrhythmias if atrioventricular nodal ablation was performed with CRT implantation or a slow ventricular rate allowed a high percentage (>90%) of biventricular pacing. CRT implantation was performed using a transvenous approach, with the right ventricular lead placed in or near the right ventricular apex. Coronary venography was performed in the left anterior oblique projection in all patients, with the fluoroscopic images divided into 4 anatomic regions (anterolateral, lateral, posterolateral, or posterior) and into equal thirds in the right anterior oblique view (basal, mid, or apical). Patients randomized to the echocardiographically guided group had LV lead placement attempted in the latest LV mechanical activation site as reported by speckle tracking echocardiography using the same segmental nomenclature used for the fluoroscopic regions. The patients randomized to the control group had their LV leads placed in the routine manner, targeting the posterior or lateral LV regions.
All echocardiographic studies (GE Vivid 7 System, Horten, Norway) were analyzed by the core laboratory at the University of Pittsburgh Medical Center Presbyterian Hospital by investigators who were unaware of all other clinical data. The LV volumes were assessed using the biplane Simpson rule and manual tracing of the digital images. For speckle tracking radial strain, digital grayscale 2-dimensional cine loop images were acquired at end-expiratory apnea from the basal and mid-LV short-axis views, with a frame rate of 60 to 90 Hz, for off-line analysis (GE EchoPac BT08-BT11). In brief, the circular regions of interest were placed on the endocardial and epicardial borders and manually adjusted for the optimal time-strain curves. The intervals to peak strain from 8 free wall segments (4 from each view) were determined from a minimum of 3 consecutive beats and averaged. The site of latest activation was determined as the segment with the latest peak strain ( Figure 1 ). The patients whose latest peak strain occurred equivalently in >1 segment were reported as such. No patient had septal or anteroseptal regions as the latest site of mechanical activation. Although a formal quantitative exclusion of scar with a predetermined cutoff was not performed, the segments with likely scar (thin wall ≤5 mm and an abnormal increase in acoustic reflectance) that had very low amplitude strain curves with significant noise were considered as missing data and, therefore, were not selected as a site of latest mechanical activation.
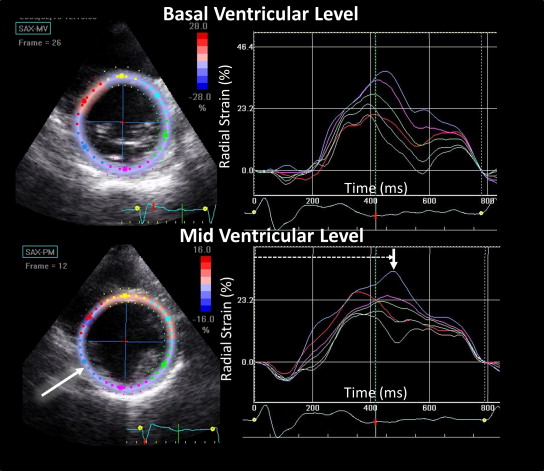
Determination of the LV lead position with respect to the site of latest mechanical activation was performed by investigators who were unaware of all patient outcome data. Polar maps using a 16-segment model of the interval to peak strain in relation to the LV lead position were constructed after device implantation. Exact concordance was defined as when the LV lead was positioned in the same segment of latest mechanical activation. Adjacent segments were defined as segments that were immediately adjacent to the latest activation site, including touching diagonally using the 11 free wall segments of the 16-segment model. The timing of the apical segments was not determined prospectively; thus, patients with apical lead positioning could only be classified as adjacent or remote.
Experienced observers who were unaware of the other patient data interpreted the baseline 12-lead electrocardiograms recorded before CRT. LBBB was defined as a QRS duration >120 ms with (1) a broad, notched R-wave in lateral precordial leads (V 5 and V 6 ) and, usually, leads I and aVL; (2) smaller or absent initial R waves in the right precordial leads (V 1 and V 2 ) followed by deep S-waves; and (3) absent Q waves in the left-sided leads. Right bundle branch block was defined as a QRS duration >120 ms with (1) broad, notched R waves (rsr′, rsR′, or rSR′ patterns) in the right precordial leads (V 1 and V 2 ); and (2) wide and deep S waves in the left precordial leads (V 5 and V 6 ). Intraventricular conduction delay was defined as conduction delay with a QRS duration >120 ms not meeting the criteria for right bundle branch block or LBBB.
The predefined primary end point was a composite of death or first HF hospitalization after CRT. Clinical events were adjudicated independently by 2 investigators not involved in patient care. The predefined secondary hard end point was a composite of death, heart transplantation, and LV assist device (LVAD) implantation. Continuous variables are presented as mean ± SD. Continuous variables from independent groups were compared using the t test and the chi-square test or Fisher’s exact test was used to compare proportions, as appropriate. Kaplan-Meier analysis was used to compare survival between patients grouped according to LV lead localization. A Cox proportional hazards model was used to assess the hazard ratios and interaction terms to evaluate the effect of QRS morphology and duration on LV lead placement benefits. Given the number of primary events, we adjusted our analyses for 2 possible confounders according to the baseline characteristics: LV ejection fraction and etiology of HF. The proportional hazards assumption was found to be adequate. A p value of <0.05 was considered significant.
Results
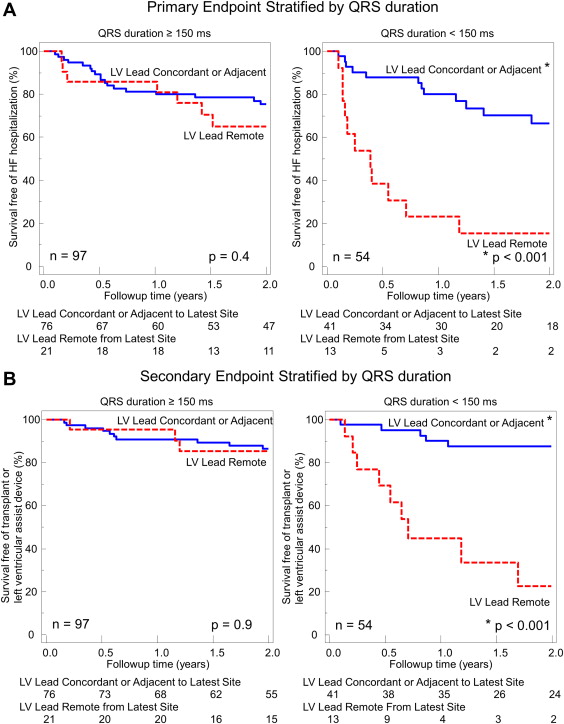
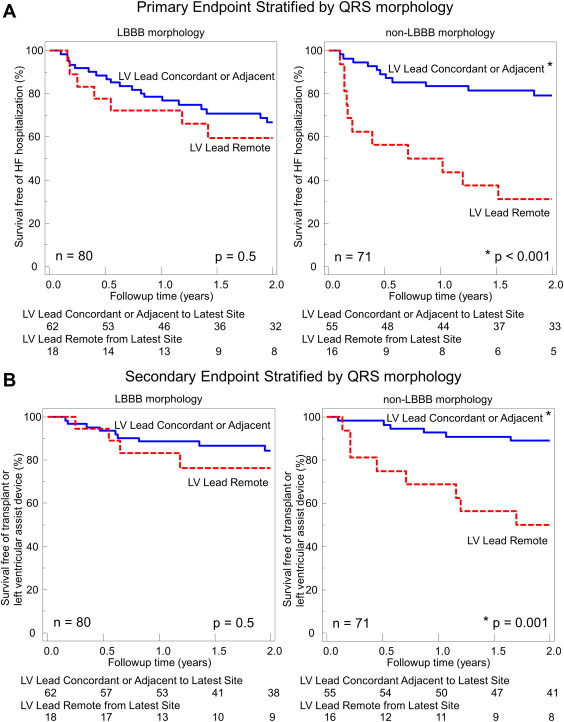
Of the 187 patients enrolled in the STARTER trial, 110 were randomized to echocardiographically guided LV lead placement and 77 to routine implantation. Most patients in both study groups belonged to New York Heart Association class III, and a high percentage of subjects were receiving β blockers (88%) and angiotensin-converting enzyme inhibitors or angiotensin receptor blockers (84%). In the echocardiographically guided group, 6 were excluded because of poor echocardiographic windows, 7 had LV lead placement failure, 1 had procedure cancellation, and 7 had uninterpretable or missing fluoroscopic and/or echocardiographic data for lead concordance analysis. In the routine group, 2 were excluded because of poor echocardiographic windows, 3 had LV lead placement failure, 3 had procedure cancellation, and 7 had uninterpretable or missing fluoroscopic and/or echocardiographic data for lead concordance analysis. Accordingly, 151 patients were included in the present substudy: 89 randomized to echocardiographically guided LV lead placement and 62 randomized to routine implantation. Overall, 97 patients had a QRS width of ≥150 ms and 54 had a QRS width <150 ms; 80 with LBBB and 71 with non-LBBB morphology ( Table 1 ). When grouped by QRS width, the patients all had similar baseline characteristics, except for the QRS width, by definition, and a higher frequency of ischemic disease in the patients with a QRS <150 ms compared with those with a QRS of ≥150 ms. When grouped by QRS morphology, the patients had similar baseline characteristics, except for the QRS duration, as expected, and a higher frequency of ischemic disease and smaller LV volumes in the non-LBBB patients than in those with LBBB. The LV lead position documented using contrast-enhanced coronary venography and biplane fluoroscopy was distributed similarly in the patient groups by QRS duration and morphology ( Table 2 ). Using the interval to peak strain in the 11 standard free wall segments combined with the biplane fluoroscopic data, 117 patients (77%) were classified as having the LV lead in the exact concordant or adjacent segment to the site of latest activation and 34 patients (23%) had the lead classified as remote from the site of latest activation. Of the 34 patients with remote lead positioning, 11 (32%) had both LBBB QRS morphology and a QRS duration of ≥150 ms, 17 (50%) had either a QRS width <150 ms or non-LBBB morphology, and 6 (18%) had both non-LBBB morphology and a QRS duration of <150 ms.
| Characteristic | Total (n = 151) | QRS Duration Groups | QRS Morphology Groups | ||||
|---|---|---|---|---|---|---|---|
| QRS ≥150 ms (n = 97) | QRS <150 ms (n = 54) | p Value | LBBB (n = 80) | Non-LBBB (n = 71) | p Value | ||
| Age (yrs) | 66 ± 12 | 66 ± 13 | 67 ± 11 | 0.46 | 65 ± 12 | 68 ± 12 | 0.061 |
| Women | 40 (26) | 28 (29) | 12 (22) | 0.38 | 26 (32) | 14 (20) | 0.076 |
| QRS width (ms) | 161 ± 28 | 177 ± 19 | 131 ± 12 | <0.01 | 156 ± 21 | 165 ± 33 | 0.045 |
| Coronary disease | 96 (64) | 55 (57) | 41 (76) | 0.019 | 45 (56) | 51 (72) | 0.047 |
| Diabetes mellitus | 52 (34) | 35 (36) | 17 (31) | 0.57 | 23 (23) | 29 (41) | 0.12 |
| Ejection fraction (%) | 25.7 ± 6.3 | 25.6 ± 6.6 | 25.9 ± 5.6 | 0.84 | 25.3 ± 6.2 | 26.2 ± 6.4 | 0.39 |
| End-diastolic volume (ml) | 191 ± 75 | 198 ± 79 | 178 ± 64 | 0.11 | 205 ± 84 | 175 ± 60 | 0.014 |
| End-systolic volume (ml) | 145 ± 63 | 150 ± 70 | 135 ± 47 | 0.15 | 155 ± 71 | 132 ± 52 | 0.027 |
| ACE inhibitors | 127 (84) | 82 (85) | 45 (83) | 0.85 | 70 (88) | 57 (80) | 0.23 |
| Spironolactone | 26 (17) | 18 (19) | 8 (15) | 0.59 | 14 (18) | 12 (17) | 0.9 |
| β Blockers | 133 (88) | 84 (87) | 49 (91) | 0.45 | 73 (91) | 60 (85) | 0.2 |
| Creatinine (mg/dL) | 1.27 ± 0.54 | 1.26 ± 0.61 | 1.28 ± 0.40 | 0.84 | 1.26 ± 0.63 | 1.29 ± 0.43 | 0.74 |
| Characteristic | Total (n = 151) | QRS Duration Groups | QRS Morphology Groups | ||||
|---|---|---|---|---|---|---|---|
| QRS ≥150 ms (n = 97) | QRS <150 ms (n = 54) | p Value | LBBB (n = 80) | Non-LBBB (n = 71) | p Value | ||
| Left anterior oblique | 0.69 | 0.87 | |||||
| Anterolateral | 9 (6) | 7 (7) | 2 (4) | 5 (6) | 4 (6) | ||
| Lateral | 60 (40) | 39 (41) | 21 (39) | 30 (38) | 30 (43) | ||
| Posterolateral | 56 (37) | 36 (38) | 20 (37) | 30 (38) | 26 (37) | ||
| Posterior | 25 (17) | 14 (15) | 11 (20) | 15 (19) | 10 (14) | ||
| Right anterior oblique | 0.91 | 0.81 | |||||
| Basal | 55 (36) | 36 (37) | 19 (35) | 28 (35) | 27 (38) | ||
| Mid | 68 (45) | 44 (45) | 24 (44) | 38 (48) | 30 (42) | ||
| Apical | 28 (19) | 17 (18) | 11 (20) | 14 (18) | 14 (20) | ||
| Lead concordance | 0.73 | 0.99 | |||||
| Concordant or adjacent ∗ | 117 (77) | 76 (78) | 41 (76) | 62 (78) | 55 (77) | ||
| Remote | 34 (23) | 21 (22) | 13 (24) | 18 (22) | 16 (23) | ||
∗ Concordant or adjacent with respect to the site of latest mechanical activation.
During a 2-year period, 48 primary events occurred in the population, including 26 HF hospitalizations, 15 deaths, 6 heart transplants, and 1 LVAD implantation. All patients with heart transplants or LVAD implantation had HF hospitalization as their primary end point, with hard events tracked as secondary events. Five patients who reached HF hospitalization as the primary end point also died later in the 2-year follow-up period, for 27 hard end points during the 2-year follow-up period. As previously reported, the overall 2-year event-free survival was 77% in the echocardiographically guided study versus 57% in the routine control group (hazard ratio [HR] 0.48, 95% confidence interval (CI) 0.28 to 0.82, p = 0.006). When the patients were grouped by QRS duration, those with ≥150 ms had significantly better event-free survival than those with a QRS duration <150 ms (HR 0.49, 95% CI 0.28 to 0.87, p = 0.014). However, patients with a QRS width <150 ms whose LV lead was concordant or adjacent to the site of latest mechanical activation had a similarly favorable primary outcome as patients with a QRS of ≥150 ms. The patient group with a QRS width <150 ms and a remote LV lead position had a very high primary event rate of HF hospitalization or death compared with the patients with concordant or adjacent lead placement (HR 5.45, 95% CI 2.36 to 12.6, p <0.001; p = 0.028 for interaction vs QRS ≥150 ms; Figure 2 ). Similarly, patients with a QRS width <150 ms, whose LV lead was concordant or adjacent to the site of latest mechanical activation, had comparatively favorable survival as patients with a QRS of ≥150 ms. However, patients with a QRS width <150 ms and a remote LV lead position had a high rate of death, transplantation, or LVAD implantation (HR 9.35, 95% CI 3.08 to 28.4, p <0.001; p = 0.014 for interaction vs QRS ≥150 ms; Figure 2 ). When grouped by QRS morphology, no significant difference was found in survival between patients with LBBB and non-LBBB (HR 1.0, 95% CI 0.58 to 1.73, p = NS). Patients with non-LBBB morphology whose LV lead was concordant or adjacent to the site of latest mechanical activation had a similarly favorable incidence of HF hospitalization or death as the patients with LBBB. The patient group with non-LBBB and a remote LV lead position had a very high primary event rate of HF hospitalization or death compared with the patients with concordant or adjacent leads (HR 4.92, 95% CI 2.12 to 11.39, p <0.001; p = 0.028 for interaction vs LBBB; Figure 3 ). Similarly, patients with non-LBBB morphology, whose LV lead was concordant or adjacent to the site of latest mechanical activation, had comparatively favorable survival as patients with LBBB. However, patients with non-LBBB with a remote LV lead position had a high rate of death, transplantation, or LVAD implantation (HR 5.70, 95% CI 1.98 to 16.5, p = 0.001; p = 0.12 for interaction vs LBBB; Figure 3 ). The comparative event rates are shown in Figure 4 . The beneficial association of concordant or adjacent LV lead positions with outcomes in patients with non-LBBB morphology and/or QRS <150 ms was mediated by the decreased survival rates in patients with remote lead locations rather than superior survival for the patients with concordant or adjacent lead positions compared with those with a QRS >150 ms or LBBB.