The problem of early recognition of atrial fibrillation (AF) is greatly aggravated by the often silent nature of the rhythm disturbance. In about 1/3 of patients with this arrhythmia, patients are not aware of the so-called asymptomatic AF. In the past 15 years, the diagnostic data provided by implanted pacemakers and defibrillators have dramatically increased knowledge about silent AF. The unreliability of symptoms to estimate AF burden and to identify patients with and without AF has been pointed out not only by pacemaker trials but also in patients without implanted devices. The technology for continuous monitoring of AF has been largely validated. It is a powerful tool to detect silent paroxysmal AF in patients without previously documented arrhythmic episodes, such as those with cryptogenic stroke or other risk factors. Early diagnosis triggers earlier treatment for primary or secondary stroke prevention. Today, new devices are also available for pure electrocardiographic monitoring, implanted subcutaneously using a minimally invasive technique. In conclusion, this recent and promising technology adds relevant clinical and scientific information to improve risk stratification for stroke and may play an important role in testing and tailoring the therapies for rhythm and rate control.
The gold standard for diagnosing atrial fibrillation (AF) is the visual inspection of the electrocardiogram. An irregular pulse may raise suspicion for AF, but an electrocardiogram is necessary to diagnose AF. The problem of early recognition of AF is greatly aggravated by the often silent nature of the rhythm disturbance. In about 1/3 of patients with this arrhythmia, patients are not aware of the so-called asymptomatic AF. Much earlier detection of the arrhythmia might allow the timely introduction of therapies to protect patients not only from the consequences of the arrhythmia but also from progression of AF from an easily treated condition to an utterly refractory problem. Not surprisingly, silent AF is associated with at least the same risk for stroke as is symptomatic AF. Patients with symptomatic AF are much more likely to be diagnosed early on and treated with stroke prevention therapy. Despite clinical evidence and guidelines, many reports from the United States and Europe have shown that only 25% to 50% of patients with AF and thromboembolic risk factors are offered antithrombotic therapies, and those who do receive therapy often discontinue it within months or do not achieve the target range of international normalized ratio values. With the advent of modern technologies in implantable cardiac devices, there is an opportunity to detect AF onset and accurately measure AF duration, frequency, burden, and ventricular rate. The availability of these new tools will open new frontiers in the scientific and clinical knowledge of AF and might have a relevant impact on patient management and risk stratification for stroke.
Limited Value of Symptoms and Intermittent Electrocardiographic Monitoring for Atrial Fibrillation Detection and Burden Assessment
The silent form of AF is noticed incidentally through a wide variety of methods, including routine physical examinations, office electrocardiography, preoperative assessments, or population surveys. In some cases, asymptomatic AF is revealed only after complications such as stroke or congestive heart failure have occurred.
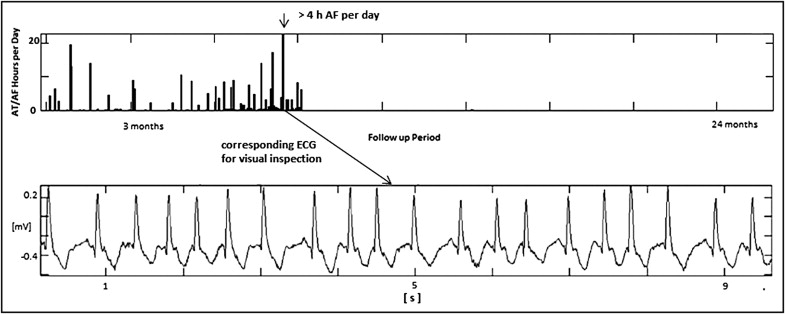
Many trials have confirmed that most paroxysmal AF (PAF) episodes are asymptomatic, many patients are completely asymptomatic, and electrocardiographic (ECG) monitoring with Holter devices has limited sensitivity. The advent of the technology for continuous AF monitoring made possible to rigorously define the AF burden as the measured percentage of time spent in AF during the follow-up period. Similarly, it was possible to rigorously define the daily AF burden as the total amount of time spent in AF each day of the follow-up period. AF burden is a parameter that takes into account the frequency and duration of AF episodes during the overall follow-up period, and daily AF burden is an estimate of the overall duration of AF episodes in each day. The trend of daily AF burden during the follow-up period is an easy and efficient way to show the presence and evolution of the disease. Moreover, it provides relevant information about the duration of recurrences ( Figure 1 ) .
The Suppression of Paroxysmal Atrial Tachyarrhythmias (SOPAT) trial showed that only 6,165 of 188,634 telephonic electrocardiograms (46%) recorded during the 1-year follow-up period were associated with specific symptoms. The remaining 54% were asymptomatic AF episodes. Similarly, the Prevention of Atrial Fibrillation After Cardioversion (PAFAC) trial collected 191,103 recordings using daily telephonic ECG monitoring; 70% of all AF recurrences occurred completely asymptomatically.
Arya et al used 7-day Holter recordings before ablation and documented AF in 92 of 114 patients (81%). All episodes were symptomatic in 35 patients (38%). In 52 patients (57%), symptomatic and asymptomatic episodes were recorded, whereas in 5 patients (5%), all documented AF episodes were asymptomatic. After ablation, the percentage of patients with only asymptomatic AF recurrences increased to 37%.
Senatore et al showed that the absence of symptoms should not be interpreted as the absence of AF, because 50% of patients were asymptomatic during ≥1 AF episode after ablation, as detected by telephonic ECG transmission.
Ziegler et al compared continuous monitoring with different strategies of intermittent ECG monitoring in pacemaker patients. All intermittent and symptom-based monitoring resulted in significantly lower sensitivity (range 31% to 71%) and negative predictive value (range 21% to 39%) for the identification of patients with AF and underestimated AF burden. These results were also confirmed by Botto et al in a similar patient population. In patients submitted to ablation and not implanted with monitoring devices, Arya et al showed that greater monitoring results in greater sensitivity for AF detection and burden estimation.
In pacemaker patients, Quirino et al showed that the sensitivity and positive predictive value of symptoms to identify AF episodes were 19% and 21%, respectively. Hanke et al used implantable subcutaneous cardiac monitors in patients submitted to surgical AF ablation and showed that sinus rhythm was documented in 53 readings of 24-hour Holter recordings but in only 34 of these instances by the implanted monitor in the time period before 24-hour Holter readings (64%), reflecting a 24-hour Holter sensitivity of 60% and a negative predictive value of only 64% for detecting AF recurrence.
In a subanalysis of the Mode Selection Trial (MOST) conducted in pacemaker patients, Glotzer et al showed that when symptoms are assessed as an indicator of atrial arrhythmias, their sensitivity to identify patients with the arrhythmia is 82.4%, but their specificity is only 38.3%, with a positive predictive value of 58.7%, making symptoms useless for risk stratification and burden assessment. Israel et al showed in pacemaker patients that AF was documented in 46% by office ECG recording compared to 88% by a review of the electrograms automatically stored by the implanted device. In addition, device interrogation revealed AF recurrences lasting >48 hours in 50 patients, 19 of whom (38%) were completely asymptomatic and in sinus rhythm at subsequent follow-up.
Pokushalov et al collected data on AF recurrences after ablation. Only 32% of electrocardiograms stored during symptomatic events corresponded to genuine AF; 39% were sinus rhythm, 19% sinus rhythm with some premature contractions, 6% sinus bradycardia, and 4% sinus tachycardia. Table 1 shows the low reliability of symptoms and ambulatory methods for monitoring AF compared with continuous monitoring through implantable devices.
| Study | Device (n) | Sinus Rhythm, AT, AF |
|---|---|---|
| Defaye et al | DDD (354) | SV arrhythmias were detected in 179 patients (50.6%), 104 of whom (65%) were asymptomatic and 117 of whom (65%) had no previous documentation. |
| Glotzer et al | DDD (312) | When symptoms were assessed as an indicator of AT/AF, their sensitivity was 82.4%, but their specificity was 38.3%, with a PPV of 58.7%. |
| Israel et al | DDD (110) | AF was documented in 51 patients (46%) by ECG recording and in 97 patients (88%) by a review of stored electrograms. AF duration >48 hours was totally asymptomatic in a significant proportion of patients. |
| Ziegler et al | DDD (574) | Intermittent (annual, quarterly, monthly 24-hour Holter; 7- and 30-day annual long-term recordings) monitoring/symptoms was highly inaccurate for identifying patients with any AT/AF. |
| Israel et al | DDD (254) | Symptoms were absent in 108 of 137 patients (79%) with device-documented AT but present in 70 of 117 patients (60%) without AT documentation. |
| Hanke et al | ICM (45) | Sinus rhythm was documented in 53 readings of 24-hour Holter monitoring but in only 34 of these instances by the implanted device, reflecting a 24-hour Holter sensitivity of 0.60 and an NPV of 0.64. |
| Botto et al | DDD (568) | The sensitivity for detecting AF episodes lasting ≥5 minutes was 44.4%, 50.4%, and 65.1% for 24-hour Holter, 1-week Holter, and 1-month Holter monitoring vs continuous monitoring. |
| Quirino et al | DDD (102) | The sensitivity and PPV of symptoms to detect AF episodes were 19% and 21%. |
| Ziegler et al | DDD (163) | Newly detected episodes of AT/AF were found on continuous monitoring in 28% of patients without previous documentation. |
| Pokushalov et al | ICM (129) | After ablation, only 32% of the symptomatic episodes corresponded to genuine AF episodes. |
Evolution in the Technology of Continuous Atrial Fibrillation Monitoring
Dual-mode, dual-pacing, dual-sensing DDD devices
Several algorithms have been studied and tested to define the optimal method for AF detection. Several trials have been conducted to validate the diagnostic and monitoring features in implantable DDD pacemakers and implantable cardioverter-defibrillators.
Pollak et al showed that by minimizing the risk for detecting artifacts caused by myopotentials or other sources of electrical interference, the detection of atrial tachyarrhythmia episodes lasting ≥5 minutes correlated well with a proved diagnosis of AF.
Many clinical studies have been conducted using the diagnostic features of implanted DDD pacemakers or implantable cardioverter-defibrillators to measure AF burden and compare different therapeutic strategies. Pacing algorithms for AF prevention have been studied using the diagnostic features of the implanted device itself. In a similar way, AF diagnostics were used to monitor AF burden in patients treated with algorithms to minimize ventricular pacing. Other studies were conducted in implanted patients to compare antiarrhythmic drug therapies in patients with PAF, through the diagnostic features of the devices.
In patients with heart failure implanted with cardiac resynchronization therapy devices, AF diagnostics have been largely used to detect new-onset AF and its progression.
Subcutaneous electrocardiographic monitors
With the advent of implantable loop recorders, a new method for detecting AF has been developed. Unlike DDD pacemakers and implantable cardioverter-defibrillators, these subcutaneous devices cannot sense endocardial atrial activity, and an analysis of consecutive RR intervals is used for the diagnosis of AF. The irregularity of the RR interval is now a proved parameter for AF detection, and the mathematical tool for the assessment is the so-called Lorentz plot ( Figure 2 ) . In a Lorenz plot, each RR interval is plotted against the previous value of the RR interval, and this can be displayed graphically and used to discriminate between AF and sinus rhythm. A recent validation study, the Reveal XT Performance Trial (XPECT), showed that a subcutaneous monitoring device equipped with an algorithm for AF detection can accurately measure AF burden (98.5%) and is very sensitive (96.4%) to identify patients with AF, independently of symptoms. The device automatically stores a 2-minute ECG strip corresponding to each of the 3 most recent episodes, thus allowing visual inspection and confirmation by the physician.
In the field of AF ablation, subcutaneous monitoring has recently been used to monitor patients after the procedure and elucidate the impact of ablation on arrhythmia recurrences. This method has been very useful to collect day-by-day data independently of patient compliance and independently of symptomatic events. Table 2 summarizes the most important technical and scientific advances on AF diagnostics using implantable devices.
| Study | Device (n) | Setting | Major Findings |
|---|---|---|---|
| Defaye et al | Chorus (617) | ED ≥1 minute | 93.8% sensitivity, 94.2% specificity for AF episode detection |
| Swerdlow et al | Jewel AF (80) | ED ≥32 VCs | 100% sensitivity, 99% specificity for AT/AF episode detection |
| Pollak et al | Thera (56) | AR ≥150 beats/min | ED ≥5 minutes had 88% correlation with true AF/AT episodes |
| Glotzer et al | DDD PMs (312) | AR ≥220 beats/min | ED ≥5 minutes had 100% sensitivity and 97% specificity for AF detection |
| Purerfellner et al | AT500 (409) | ED ≥24 VCs | 100% of the sustained atrial arrhythmia episodes detected |
| Hoffmann et al | Selection (98) | AR ≥200 beats/min, ED ≥6 VCs | Detailed analysis of rate and rhythm changes before AF onset |
| Pérez et al | Selection (282) | AR ≥200 beats/min, ED ≥10 VCs | Time to the first AF recurrence was not a surrogate of AF burden |
| Nowak et al | Pulsar M (351) | AR ≥170 beats/min, ED ≥4 VCs | Therapeutic decisions based on validated stored electrograms |
| Martinek et al | AT500 (41) | AC <360 ms, ED ≥24 VCs | Continuous monitoring was significantly more sensitive than follow-up |
| Hanke et al | Reveal XT (45) | 2-minute Lorentz plot | Continuous monitoring was superior to any conventional strategy |
| Hindricks et al | Reveal XT (247) | 2-minute Lorentz plot | Overall accuracy of Reveal XT for detecting AF was 98.5% |
Evolution in the Technology of Continuous Atrial Fibrillation Monitoring
Dual-mode, dual-pacing, dual-sensing DDD devices
Several algorithms have been studied and tested to define the optimal method for AF detection. Several trials have been conducted to validate the diagnostic and monitoring features in implantable DDD pacemakers and implantable cardioverter-defibrillators.
Pollak et al showed that by minimizing the risk for detecting artifacts caused by myopotentials or other sources of electrical interference, the detection of atrial tachyarrhythmia episodes lasting ≥5 minutes correlated well with a proved diagnosis of AF.
Many clinical studies have been conducted using the diagnostic features of implanted DDD pacemakers or implantable cardioverter-defibrillators to measure AF burden and compare different therapeutic strategies. Pacing algorithms for AF prevention have been studied using the diagnostic features of the implanted device itself. In a similar way, AF diagnostics were used to monitor AF burden in patients treated with algorithms to minimize ventricular pacing. Other studies were conducted in implanted patients to compare antiarrhythmic drug therapies in patients with PAF, through the diagnostic features of the devices.
In patients with heart failure implanted with cardiac resynchronization therapy devices, AF diagnostics have been largely used to detect new-onset AF and its progression.
Subcutaneous electrocardiographic monitors
With the advent of implantable loop recorders, a new method for detecting AF has been developed. Unlike DDD pacemakers and implantable cardioverter-defibrillators, these subcutaneous devices cannot sense endocardial atrial activity, and an analysis of consecutive RR intervals is used for the diagnosis of AF. The irregularity of the RR interval is now a proved parameter for AF detection, and the mathematical tool for the assessment is the so-called Lorentz plot ( Figure 2 ) . In a Lorenz plot, each RR interval is plotted against the previous value of the RR interval, and this can be displayed graphically and used to discriminate between AF and sinus rhythm. A recent validation study, the Reveal XT Performance Trial (XPECT), showed that a subcutaneous monitoring device equipped with an algorithm for AF detection can accurately measure AF burden (98.5%) and is very sensitive (96.4%) to identify patients with AF, independently of symptoms. The device automatically stores a 2-minute ECG strip corresponding to each of the 3 most recent episodes, thus allowing visual inspection and confirmation by the physician.




