Patients with chronic heart failure (CHF) at risk of sudden cardiac death (SCD) are often treated with implantable cardiac defibrillators (ICDs). However, current criteria for device use that is based largely on left ventricular ejection fraction (LVEF) lead to many patients receiving ICDs that never deliver therapy. It is of clinical significance to identify patients who do not require ICDs. Although cardiac I-123 meta-iodobenzylguanidine (MIBG) imaging provides prognostic information about CHF, whether it can identify patients with CHF who do not require an ICD remains unclear. We studied 81 patients with CHF and LVEF <35%, assessed by cardiac MIBG imaging at enrollment. The heart-to-mediastinal ratio (H/M) in delayed images and washout rates were divided into 6 grades from 0 to 5, according to the degree of deviation from control values. The study patients were classified into 3 groups: low (1 to 4), intermediate (5 to 7), and high (8 to 10), according to the MIBG scores defined as the sum of the H/M and washout rate scores. Sixteen patients died of SCD during a follow-up period. Patients with low MIBG score had a significantly lower risk of SCD than those with intermediate and high scores (low [n = 19], 0%; intermediate [n = 37], 19%; high [n = 25], 36%; p = 0.001). The positive predictive value of low MIBG score for identifying patients without SCD was 100%. In conclusion, the MIBG score can identify patients with CHF and LVEF <35% who have low risk of developing SCD.
Despite recent advances in pharmacological and nonpharmacological therapies, the mortality rate of patients with chronic heart failure (CHF) remains high because of sudden cardiac death (SCD). Recent clinical trials have shown that implantable cardioventricular defibrillators (ICDs) could reduce the risk of SCD in patients with CHF who have low left ventricular ejection fraction (LVEF). Therefore, many guidelines recommend ICD implantation for the primary prevention of SCD based on LVEF. However, numerous patients with ICDs implanted have never actually received ICD therapies. Therefore, alternative means are needed to refine the qualification criteria for ICD implantation and to identify patients with CHF who have reduced LVEF but do not require ICD implantation, which is clinically important. Cardiac imaging with I-123 meta-iodobenzylguanidine (MIBG) can predict poor clinical outcomes, including SCD. However, stratifying patient with CHF based on MIBG imaging has not been fully evaluated. The present study determines whether MIBG imaging can identify patients with CHF who are at low risk for SCD and do not require an ICD.
Methods
We enrolled 81 consecutive outpatients with stable CHF diagnosed based on clinical signs and symptoms according to the Framingham criteria, no sustained ventricular tachycardia, and LVEF <35% determined using radionuclide angiography. None of the patients had an ICD, biventricular pacemaker, or biventricular defibrillator at enrollment. The exclusion criteria comprised significant renal dysfunction, insulin-dependent diabetes mellitus, and autonomic neuropathy.
At the time of entry into the study, all patients had been assessed by cardiac MIBG imaging, echocardiography, and Holter electrocardiography, and venous blood samples were withdrawn from all of them. The Osaka General Medical Center Review Committee study approved the protocol, and all patients provided written informed consent to participate.
All patients were assessed while resting in the supine position by myocardial imaging with I-123 MIBG (Fuji Film RI Pharma Laboratory, Tokyo, Japan) using the same gamma camera as that used for radionuclide angiography. The patients were injected intravenously with 111-MBq of I-123 MIBG after an overnight fast. Initial and delayed images were acquired in the anterior chest view at 20 and 200 minutes later, respectively. Two independent observers who were unaware of the clinical status of patients assessed cardiac MIBG uptake as described. The heart-to-mediastinal ratio (H/M) in early (H/M(e)) and delayed (H/M(d)) images was then calculated by dividing the mean counts/pixel in the left ventricle by those in the mediastinum with background subtraction. The radioactive decay of I-123 was taken into consideration, and then the cardiac MIBG washout rate (WR) was calculated from the initial and delayed images as described. Table 1 lists how we graded the H/M(d) and WR based on the degree of deviation from control values (H/M, 2.65 ± 0.32; WR, 9.6 ± 8.5%), respectively. The MIBG score was defined as the sum of the H/M(d) and WR scores. The patients were classified as having low (1 to 4) intermediate (5 to 7) or high (8 to 10) MIBG scores.
| Score | Mean H/M(d) | Mean WR(%) |
|---|---|---|
| 0 | 2.65 – | –9.6 |
| 1 | 2.33 ∗ – 2.64 | 9.7–18.1 ll |
| 2 | 2.01 † – 2.32 | 18.1–26.6 ¶ |
| 3 | 1.69 ‡ – 2.00 | 26.7–35.1 # |
| 4 | 1.37 § – 1.68 | 35.2–43.6 ∗∗ |
| 5 | –1.36 | 43.7– |
Left ventricular end-diastolic dimension and left atrial dimension were measured by 2-dimensional echocardiography using the standard techniques. Ventricular arrhythmias assessed by 24-hour electrocardiographic monitoring were classified according to Lown’s grade, and nonsustained ventricular tachycardia was defined as ≥5 consecutive premature ventricular beats lasting <30 seconds. Plasma noradrenaline and serum uric acid and sodium and creatinine concentrations were measured in blood samples collected through an intravenous cannula inserted into patients who had rested for at least 30 minutes in the supine position. Noradrenaline concentration in plasma samples mixed with ethylenediaminetetraacetic acid was determined by high-performance liquid chromatography at Shionogi Biomedical Laboratories (Osaka, Japan).
All study patients were followed up prospectively at our institution for at least 5 years by clinicians who were blinded to the cardiac MIBG imaging results. The primary end point was SCD, defined as witnessed cardiac arrest or death within 1 hour of the onset of acute symptoms, unexpected or unwitnessed death in a patient who was known to have been well within the previous 24 hours. The secondary end point comprised pump failure death (PFD), defined as death because of progressively reduced cardiac output and failed organ perfusion.
Data are shown as mean ± SD. Differences among continuous and discrete variables were, respectively, compared using Student’s t test and Fisher’s exact test in patients with and without cardiac events. Cardiac event-free rates were calculated using the Kaplan-Meier method, and differences between each group were detected using the log-rank test. Variables that predicted outcomes in univariate analyses (p <0.05) were entered into multivariate analyses, and their significance as independent predictors of cardiac events was determined using the Cox proportional hazards regression model. The sensitivity, specificity, predictive accuracy, and positive and negative predictive values among the various criteria for predicting cardiac events were compared using Fisher’s exact test. All data were statistically analyzed using EZR, (Saitama Medical Center, Jichi Medical University, Saitama, Japan) version 1.03. p Values <0.05 were considered statistically significant differences.
Results
Clinical characteristics of the study patients were listed in Table 1 . All patients were followed up for at least 5 years. Thirty-eight of them died during a mean follow-up of 6.9 ± 3.1 years and 27 of them died of cardiac causes, the most prevalent being SCD (n = 16), followed by PFD (n = 11).
Table 2 and Figure 1 show the baseline characteristics and MIBG imaging results of patients with and without SCD, respectively. Patients with SCD had a significantly lower LVEF and H/M(d) and significantly higher WR and MIBG scores than those without SCD. The characteristics of patients with low, intermediate, and high MIBG score are also listed in Table 2 .
| Variables | All patients (n=81) | Sudden cardiac death | p Value | MIBG score | p Value † | |||
|---|---|---|---|---|---|---|---|---|
| Yes (n=16) | No (n=65) | low (n=19) | Intermediate (n=37) | High (n=25) | ||||
| Age (years) | 63±13 | 62±13 | 64±13 | n.s. | 64±12 | 62±13 | 64±11 | n.s. |
| Men | 62 (77%) | 12 (75%) | 50 (77%) | n.s. | 15 (79%) | 26 (70%) | 21 (84%) | n.s. |
| Weight (kg) | 59±13 | 58±12 | 60±13 | n.s. | 58±10 | 60±14 | 60±12 | n.s. |
| NYHA class | 2.1±0.6 ‡ | 2.4±0.6 | 2.1±0.6 | n.s. | 2.0±0.6 | 2.1±0.6 | 2.4±0.6 | n.s. |
| Heart rate (beats/min) | 74±12 | 73±12 | 75±12 | n.s. | 77±14 | 73±11 | 73±10 | n.s. |
| Systolic pressure (mmHg) | 126±17 | 127±12 | 125±18 | n.s. | 129±17 | 125±17 | 124±16 | n.s. |
| Ischemic heart disease | 39 (48%) | 6 (38%) | 33 (51%) | n.s. | 11 (58%) | 19 (51%) | 9 (36%) | n.s. |
| Medications | ||||||||
| Beta-blocker ∗ | 57 (67%) | 11 (69%) | 46 (71%) | n.s. | 14 (73%) | 28 (76%) | 15 (60%) | n.s. |
| Diuretics | 58 (71%) | 13 (81%) | 45 (69%) | n.s. | 10 (53%) | 25 (68%) | 23 (92%) | n.s. |
| ACE inhibitors/ARB | 69 (85%) | 13 (81%) | 56 (86%) | n.s. | 15 (79%) | 30 (81%) | 24 (96%) | n.s. |
| Aldosterone blocker | 52 (64%) | 10 (63%) | 42 (65%) | n.s. | 10 (53%) | 24 (57%) | 18 (72%) | n.s. |
| Radionuclide angiography | ||||||||
| LV ejection fraction (%) | 26%±6 | 24±7 | 27±6 | 0.03 | 28±5 | 26±7 | 26±5 | n.s. |
| Echocardiography | ||||||||
| Left atrial dimension (mm) | 43±8 | 45±8 | 43±8 | n.s. | 40±6 | 43±8 | 46±9 | n=0.03 |
| Left ventricular end-diastolic dimension (mm) | 63±8 | 64±5 | 63±9 | n.s. | 60±7 | 63±8 | 66±9 | n=0.03 |
| Holter electrocardiography | ||||||||
| Lown’s grade | 3.5±1.3 | 3.9±1.2 | 3.4±1.4 | n.s. | 2.8±1.8 | 3.7±1.2 | 3.9±0.9 | n=0.003 |
| Non-sustained ventricular tachycardia | 15 (19%) | 3 (19%) | 12 (18%) | n.s. | 3 (16%) | 7 (19%) | 5 (20%) | n.s. |
| Norepinephrine (pg/mL) | 466±252 | 599±267 | 434±240 | 0.02 | 413±210 | 417±218 | 580±299 | n.s. |
| Sodium (mmol/L) | 136±3.1 | 137±3.7 | 139±2.9 | n.s. | 140±2.3 | 140±2.8 | 137±3.4 | n.s. |
| Creatinine (mg/dL) | 0.93±0.26 | 0.92±0.32 | 0.92±0.25 | n.s. | 0.88±0.16 | 0.89±0.25 | 1.03±0.31 | n.s. |
| Uric acid (mg/dL) | 6.9±1.8 | 7.8±1.8 | 6.7±1.8 | 0.03 | 6.1±1.7 | 6.9±1.8 | 7.5±2.0 | n=0.03 |
∗ Ten, 50 and 21 of the patients were in NYHA functional classes I, II and III, respectively.
† Medication with beta-blocker (carvedilol) scored at the last follow-up.
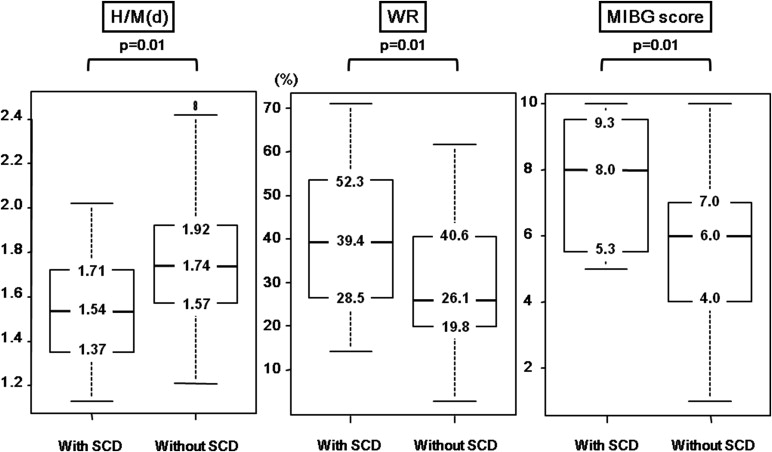
Multivariate Cox analysis revealed that the MIBG score was significantly and independently associated with SCD (p = 0.012), although univariate analysis associated H/M(d) and WR with poor outcomes ( Table 3 ). The MIBG score was significantly associated (p = 0.008) with PFD independently of LVEF and serum creatinine levels.
| Variables | Univariate | Multivariate | ||
|---|---|---|---|---|
| p | HR (95% CI) | p | HR (95% CI) | |
| Cox model for SCD | ||||
| MIBG score | 0.0011 | 4.120 (1.758-9.657) | 0.012 | 3.26 (1.39-8.27) |
| WR | 0.0033 | 1.048 (1.016-1.081) | … | … |
| H/M(d) | 0.0059 | 0.071 (0.011-0.478) | … | … |
| Uric acid | 0.0073 | 1.479 (1.111-1.969) | 0.048 | 1.35 (1.00-1.83) |
| Norepinephrine | 0.0259 | 1.002 (1.000-1.003) | … | … |
| Left ventricular end-diastolic dimension | 0.0417 | 0.925 (0.858-0.997) | … | … |
| Cox model for PFD | ||||
| WR | 0.0010 | 1.068 (1.027-1.111) | … | … |
| MIBG score | 0.0018 | 5.872 (1.925-17.92) | 0.008 | 5.69 (1.56-20.7) |
| Creatine | 0.0033 | 11.20 (2.236-56.09) | 0.011 | 8.85 (1.65-47.6) |
| Uric acid | 0.0060 | 1.652 (1.155-2.364) | … | … |
| Left ventricular end-diastolic dimension | 0.0037 | 1.079 (1.025-1.136) | … | … |
| Left ventricular ejection fraction | 0.0294 | 0.904 (0.825-0.990) | 0.044 | 0.89 (0.79-1.00) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


