Fig. 51.1
Left atrial appendage histology slide depicting thin wall between pectinate ridges. The LAA is a very thin-walled structure as depicted here; the tissue between the pectinate ridges is less than 400 μm in thickness accounting for the ease of inadvertent perforation and pericardial effusion during instrumentation (Adapted with permission from Ref. [19])
Though traditionally thought to serve no cardiac function, the LAA generates atrial natriuretic peptide and contributes to compliance of the left atrium [21]. The LAA is also electrically active, and the source of non-pulmonary vein atrial fibrillation triggers in 32 % of patients >80 years old [22]. Amputation or ablation of the LAA may thus decrease the overall burden of atrial fibrillation in some patients.
51.3 Left Atrial Appendage Closure
Closure of the LAA was first explored in the 1940s, but interest waned with the development of vitamin K antagonists. More recently interest resurfaced and has been explored initially with surgical approaches performed in conjunction with concomitant heart surgeries. As is often the case with surgical technique developments, the data are limited to case series and retrospective analyses with the notable exception of the Left Atrial Appendage Occlusion Study II (LAAOS II). Three surgical devices, the AtriClip (AtriCure, West Chester, OH), TigerPaw (Maquet, Rastatt, Germany), and Endoloop suture (Johnson and Johnson, Cincinnati, OH), have been developed and tested. Percutaneous LAA closure has been an attractive idea and prompted the development of multiple devices which can broadly be categorized as “plugs” which are placed internally occluding the appendage at the ostium and “ligatures” which close the appendage from the epicardium by cinching around the ostium. Data with percutaneous devices has been slow to accumulate given slow regulatory approval and is dominated by the relatively large randomized trials of the Watchman device (Boston Scientific, Plymouth, MN) (see Table 51.1 and Fig. 51.3).
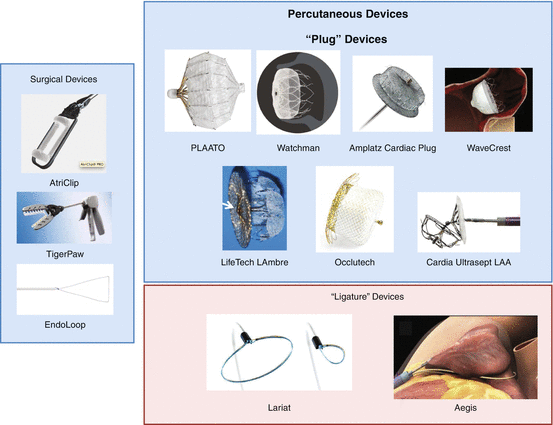
Table 51.1
Left atrial appendage devices
Device | Manufacturer | Comment |
|---|---|---|
Surgical | ||
AtriClip | AtriCure | Clip device closes base of LAA. 95 % success in the initial trial |
TigerPaw | LAAx Inc. | Creates mattress suture closure of LAA. 93 % success in the initial trial |
Endoloop snare | Johnson & Johnson | 0 Prolene ligature. 75 % had residual leak by CT |
Percutaneous – plug | ||
PLAATO | ev3 Inc. | First percutaneous device tested in trials. No longer in production |
Watchman | Boston Scientific | Only device subjected to prospective randomized trials in comparison to warfarin |
Amplatz cardiac plug | St. Jude Medical | Shallow profile to allow deployment in multiple lobed LAA |
WaveCrest | Coherex Medical | Shallow with most retention barbs |
Occlutech | Occlutech | No retention barbs to minimize risk of LAA perforation. Relies on loops to hold LAA trabeculae |
LifeTech LAmbre | LifeTech | Hinged ball to facilitate placement orthogonal to LAA ostium |
Cardia Ultrasept LAA | Cardia Inc. | Ball hinge between anchor and sealing membrane to increase conformity |
Epicardial | ||
Lariat | SentreHeart Inc. | Magnetic rail created between endocardial and epicardial surfaces. Clinically approved in US without randomized data |
Aegis | Aegis Medical | Loop snare with no endocardial component. Monitors for cessation of LAA electrical activity |

Fig. 51.3
LAA closure devices. Devices are categorized as surgical or percutaneous, and percutaneous devices are of two general styles – “plugs” which fill and occlude the LAA from within and “ligatures” which cinch and occlude the LAA from the outside
51.4 Surgical LAA Closure
Surgical closure of the LAA is widely performed with a concomitant cardiac surgical procedure, whether bypass grafting and valve repair or replacement and often in conjunction with a surgical Cox maze procedure. Surgical techniques have been detailed elsewhere and include suture exclusion, stapled exclusion, and amputation with oversewing [23].
Initial retrospective analyses of patient having undergone surgical LAA closure were positive. In one study of 205 high-risk patients undergoing mitral valve replacement (predominantly for rheumatic disease), 58 had surgical LAA closure which was associated with a significantly reduced risk of thromboembolic event (17 % vs. 34 %) [24]. Notably, incomplete LAA closure was noted in six patients (10 %), which was associated with an increased risk of thromboembolism. The Left Atrial Appendage Occlusion Study (LAAOS) intended to study the safety of surgical LAA closure and prospectively randomized 77 patients with atrial fibrillation and risk factors undergoing CABG (+/− valve surgery) to LAA ligation versus control (52 and 25, respectively) [25]. The study was not powered for efficacy, and a learning curve was demonstrated with improvements in successful closure after performing four procedures. A high rate of incomplete closure was noted (55 % of suture-mediated closures and 28 % of staple-mediated closures). The high rate of incomplete surgical closure was substantiated in a retrospective study of 137 patients over 10 years who had undergone surgical LAA closure with subsequent TEE at the Cleveland Clinic [26]. Surgical closure was successful in 40 % of the time, and unsuccessful closure was associated with thrombus in 41 % of cases but none with excision. Similarly, among 94 patients at the Mayo Clinic (Minnesota) with surgical LAA closure who underwent TEE for postoperative AFib, new thrombus was seen in 28 %, and seen significantly more often when the LAA was incompletely closed (47 % vs. 12 %, P = 0.002), and LAA amputation was associated with significantly less residual patency than ligation (51 % vs. 17 %, P < 0.0001) [27]. LAA amputation was employed as a strategy in the LAAOS II trial in 92 % of cases when complete occlusion was required by the study protocol [28]. Amputation of the LAA appears to be a superior strategy of surgical LAA closure when compared to ligation which is associated with a significant rate of incomplete closure. Moreover, incomplete LAA closure is consistently associated with increased risk of thrombus formation [29].
51.5 Surgical Devices
Three surgical devices have been developed for LAA closure; however, data remains limited. The AtriClip device (AtriCure, West Chester, OH) is a mechanical clip surgically placed under direct visualization at the base of the LAA. In the US experience, it successfully occluded the LAA in 67 of 70 (95 %) patients, and the LAA remained occluded in 98 % of patients at 3 months [30]. There were no device-related adverse events.
The TigerPaw device (LAAx Inc., Livermore, CA acquired by Maquet, Rastatt, Germany) is likewise surgically placed under direct visualization. It consists of a series of male and female U-shaped barbs buttressed by a soft silicone ring intended to form mattress suture-style closure at the base of the LAA. In the initial study of 60 implants, there was a single device-related adverse event (minor tissue tear requiring suture), and complete closure (by TEE) was noted acutely in 56 (93 %) and in all 54 who had 90-day follow-up imaging [31].
Finally, the Endoloop suture (Johnson & Johnson, Cincinnati, OH) is an 0 Prolene ligature secured at the base of the LAA to close it off. It was initially tested in 12 patients without any immediate procedural complications, yet in 9 (75 %) there was residual patency by postoperative CT assessment [32].
51.6 Percutaneous LAA Closure
Percutaneous closure of the LAA is an attractive idea for multiple reasons. Many patients have atrial fibrillation with accompanying stroke risk, and the LAA has been established as the predominant location of thrombus formation in the left atrium. While surgical LAA amputation is generally effective, most patients with atrial fibrillation do not have concomitant need for cardiac surgery, so a low-risk percutaneous procedure to accomplish the same effect is desirable particularly in an older, frail population. Moreover, while anticoagulation effectively reduces stroke risk in AF, the bleeding risk of oral anticoagulation is cumulative, may be prohibitive for some patients, and augmented by concomitant use of antiplatelet agents, so a device or procedure is sought which could reduce stroke risk while obviating the need for anticoagulation.
Percutaneous devices for LAA closure include “plugs” and “ligatures.” All percutaneous devices are placed in a cardiac catheterization laboratory using a combination of fluoroscopic and transesophageal (TEE) guidance. The use of TEE guidance during the procedure offers the benefit of ensuring adequate LAA closure and appropriate device positioning before concluding the case. All approaches require adequate anticoagulation (usually with unfractionated heparin), while any device is in the left atrium; however, management of peri-procedural anticoagulation and antiplatelet medicines have varied across devices and trials and may be a source of disparity in outcomes (e.g., access site bleeding and peri-procedural stroke).
The “plug” devices are all endocardial and placed via transvenous transseptal approach using combined fluoroscopic and transesophageal imaging guidance. No LAA closure device has been approved for clinical use in the United States. The devices which have been studied in humans or approved for clinical use in Europe (CE Mark approval) include the PLAATO device (Appriva Medical, Sunnyvale, CA – removed from market), the Watchman device (Boston Scientific, Maple Grove, MN), the WaveCrest device (Coherex Medical, Salt Lake City, UT), and the Amplatz Cardiac Plug (St. Jude Medical, St. Paul, MN).
The first widely studied percutaneous LAA closure device was the PLAATO device (Appriva Medical, Sunnyvale, CA). It is composed of a self-expanding nitinol frame with retention barbs wrapped in an impermeable membrane. Placement involved unsheathing the device within the ostium of the LAA using a 12F delivery sheath. Preclinical studies revealed subsequent endothelial ingrowth sealing off the opening to the appendage. Patients were treated with aspirin and clopidogrel but not anticoagulation before and following the procedure.
The early clinical experience was collated from feasibility trials in multiple centers in Europe and North America. In 111 patients with non-valvular atrial fibrillation, an average CHADS2 score of 2.5, and a contraindication to anticoagulation, device placement was successful in 108 (97.3 %). A single patient died, and two patients suffered stroke during an average of 9.8 months of follow-up. The two strokes (2.2 % per patient-year) compared favorably with the 6.3 % per year predicted stroke rate based on the study population risk profile. Five patients (4.5 %) developed pericardial effusion which was without sequelae in four of the five. The patient who died developed hemopericardium following transseptal puncture (before device deployment) and required emergent cardiac surgery. This patient’s recovery was complicated by DVT, intracranial hemorrhage on anticoagulation, and subsequent death [33]. The authors concluded percutaneous LAA closure could be performed reliably with acceptable risk.
Five-year follow-up was possible in 64 patients from the North American experience (the European feasibility trial required only 1-year follow-up). The study group experienced a stroke/TIA rate of 3.8 % per year, a 42 % reduction compared to the 6.6 % per year rate predicted [34]. Similarly in the European experience of 180 patients who underwent PLAATO device placement, the annualized observed stroke rate of 2.3 %/year over 129 patient-years was less than the 6.6 %/year predicted rate [35]. Device placement was successful 90 % of the time, and there were eight procedure-related major adverse events (MAEs). Despite these initially promising results, the PLAATO device was withdrawn from the market and further study.
The Watchman device (Boston Scientific) has been the most extensively studied percutaneous device for LAA closure. Similar to the PLAATO device, it is made of a self-expanding nitinol wire frame with retention barbs and covered with a PET membrane. Delivery is via a 14F sheath with the device unsheathed in the LAA ostium. It is available in five sizes from 21 to 33 mm diameter with recommended 20 % oversizing. The procedure is performed with TEE and fluoroscopic guidance, and patients are treated with aspirin and clopidogrel peri-procedurally with oral anticoagulation for 45 days following implantation until TEE verification of complete closure. Patients without leak around the device (<5 mm of flow by TEE) are then switched to aspirin and clopidogrel for the balance of 6 months at which point aspirin alone is continued indefinitely.
The Percutaneous Closure of the Left Atrial Appendage versus Warfarin Therapy for Prevention of Stroke in Patients with Atrial Fibrillation (PROTECT-AF) trial and the Prospective Randomized Evaluation of the Watchman Left Atrial Appendage Closure Device in Patients with Atrial Fibrillation versus Long-Term Warfarin Therapy (PREVAIL) trial of the Watchman device are the only prospective randomized trials of percutaneous LAA closure. The PROTECT-AF was a non-inferiority trial of 707 patients with atrial fibrillation and stroke risk and who were eligible for anticoagulation. Patients were randomized 2:1 (463 device, 244 control/warfarin) and followed initially for 1,065 patient-years [36]. Non-inferiority was met and maintained out to 1,500 patient-years of follow-up [37]. The composite primary efficacy endpoint (stroke, death, or systemic embolism) was less (3.0 per 100 patient-years) in the device arm compared to the control arm (4.9 per 100 patient-years, >99.9 % for non-inferiority). In the successfully treated population, the results were even better (1.9 vs. 4.6 per 100 patient-years). Surprisingly, cardiovascular death was 74 % lower in the device group (0.7 vs. 2.7 per 100 patient-years) than the control group (see Fig. 51.4). Moreover, the device group experienced a 94 % reduction in hemorrhagic stroke (0.1 vs. 1.6 per 100 patient-years). As expected, the control group experienced more major bleeding (4.1 %) and hemorrhagic stroke (2.5 %), while the device group experienced more procedure-related complications (pericardial effusion 4.8 %, major bleeding 3.5 %, device embolization 0.6 %, procedure-related ischemic stroke 1.1 %). The overall increased risk of procedural complication was offset over time by the reduction in hemorrhagic stroke relative to the anticoagulated control arm. Notably, the observed rate of ischemic stroke in the control arm (1.6 per 100 patient-years) was lower than that predicted by their risk profile. In this study, 86 % of patients were able to stop anticoagulation at 45-day follow-up, which rose to 92 % at 6 months. In the continued access protocol (CAP) registry data of 460 patients with device implants following PROTECT-AF, the rate of procedural complication by experienced operators improved substantially (2.2 % pericardial effusion, 0 % peri-procedural stroke) [38]. Sub-analyses from the PROTECT-AF data revealed a significant improvement in quality of life following device placement and no increased risk related to unclosed iatrogenic atrial septal defects [39, 40]. The overall results of the PROTECT-AF and CAP data were positive suggesting a role for device therapy in stroke reduction for patients with non-valvular atrial fibrillation.
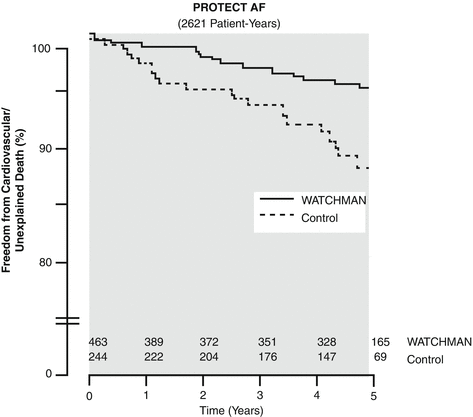

Fig. 51.4
Mortality benefit of the Watchman device. In randomized trial data patients who received the Watchman device had a lower mortality than those continued on standard therapy (Data prepared by Boston Scientific for FDA Panel meeting December 11, 2013. Available at http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/MedicalDevices/MedicalDevicesAdvisoryCommittee/CirculatorySystemDevicesPanel/UCM377935.pdf. Accessed Nov 4, 2014)
Nevertheless, concern remained over the safety and efficacy profiles of the device prompting the PREVAIL trial which randomized 407 patients at 41 US centers in the same 2:1 fashion and with the same medication plan as PROTECT-AF [41]. Importantly, inclusion criteria for PREVAIL were revised to a higher CHADS2 score of 2 or more (vs. 1 or more) targeting a higher-risk population than in PROTECT-AF (avg. CHADS2 score 2.6 vs. 2.2). The trial was required to include new sites and new operators to test the durability of the safety profile; thus 39 % of implants were performed by new operators. Nevertheless, implant success was 95 % (improved from 90 % in PROTECT-AF), while procedural complication rate was significantly lower than previously documented (4.2 % vs. 8.7 % overall). There were no peri-procedural strokes. Pericardial effusions requiring surgery decreased significantly (1.6–0.4 %, P = 0.027), and 98 % of patients could discontinue warfarin at 6 months (up from 92 % in PROTECT-AF). The trial was marked by a low event rate in the control arm, which experienced a single ischemic stroke and no hemorrhagic strokes. Overall, the pre-specified statistical endpoint for non-inferiority was not met; however, the co-primary endpoints and safety endpoints were met supporting the safety and efficacy of the Watchman device even with novice implanters.
All trials involving the Watchman device required patient eligibility to take oral anticoagulation; however, a major interest in device-based stroke reduction in atrial fibrillation is for patients who cannot be safely anticoagulated. Thus the ASA Plavix Feasibility Study with Watchman Left Atrial Appendage Closure Technology (ASAP) sought to address the use of the Watchman device in patients who were ineligible for warfarin [42]. A total of 150 patients with an average CHADS2 score of 2.8 were prospectively enrolled and treated with aspirin plus 6 months of clopidogrel. There were six cases of device-related thrombus, but only one associated with stroke giving an annualized device thrombus-related stroke rate of 0.3 % per year. The observed overall stroke rate of 1.7 % per year represents a 77 % reduction compared with the expected rate of 7.3 % per year based on the CHADS2 score.
Importantly, the vast majority of patient who receive a Watchman device were able to stop warfarin, and this percentage increased with increased experience in device placement and patient selection. In the most recent study, PREVAIL, 99 % of patients who received a Watchman device were able to stop taking warfarin (see Table 51.2).
Table 51.2
Warfarin cessation in Watchman trials
Visit | PROTECT-AF (N = 408) | PREVAIL (N = 252) | CAP (N = 566) |
|---|---|---|---|
45 day | 87 % | 92 % | 96 % |
6 month | 92 % | 98 % | 99 % |
12 month | 93 % | 99 % | 96 % |
The Amplatz Cardiac Plug (St. Jude Medical) is another percutaneous transcatheter device for LAA closure. It is composed of two woven nickel-titanium self-expanding discs with retention barbs on the distal disc and a polyester patch embedded within the discs. The distal disc is placed within the LAA and holds the proximal disc flush against the ostium. Aspirin plus clopidogrel is used peri-procedurally but not oral anticoagulation. The early European experience with the device was summarized retrospectively by Park et al. [43]. The experience involved 137 patients who underwent procedure with a 96 % (132 of 137) success rate and 7 % (10 of 137) serious complication rate [43]. In a prospective Asia-Pacific study, 20 patients ineligible for warfarin underwent device implant with 95 % success. No stroke or death occurred during the 12-month follow-up [44].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


