Use of Mechanical Devices in Treating Heart Failure
Timothy J. Myers
Reynolds M. Delgado III
O. H. Frazier
Congestive heart failure (CHF) is the most common cause of death in the industrialized world. Current medical therapy can relieve its symptoms (1) and can even prolong life in some cases (2,3). Unfortunately, the relentless nature of the underlying disease process means that CHF almost always proves fatal in the end. When conventional therapy fails to alleviate end-stage heart failure, mechanical circulatory support (MCS) is a possible option. During the past several decades, MCS has become an effective means of saving many patients who would otherwise die of progressive CHF.
Today physicians can choose from a wide range of MCS systems, depending on the desired degree of support, length of support, and extent of postoperative mobility. Short-term MCS may be used to maintain patients who develop acute, reversible heart failure after a myocardial infarction or open heart surgery. Longer-term MCS may be applied to patients with severe acute and chronic heart failure, including those awaiting heart transplantation. With prolonged (>30-day) support, transplant candidates may undergo cardiac rehabilitation, which often reverses cardiac and end-organ dysfunction, improving the patient’s operative status (4,5).
MCS has evolved, somewhat in parallel with cardiac transplantation, toward the goal of lowering the morbidity and mortality of CHF. By combining these two interventions, physicians are salvaging many otherwise hopeless cases. MCS is now being used as destination therapy. According to the Institute of Medicine, MCS could benefit some 35,000 to 70,000 Americans per year (6).
After briefly describing the history of MCS systems, this chapter will focus first on the major devices currently being used for short-term support, and then on implantable systems being used for longer-term bridging to transplantation and destination therapy.
History
Although the use of mechanical devices to support the human heart has become prevalent only within the last decade, the concept of MCS has a long history. Nearly two centuries ago, LeGallois (7) speculated that temporary or permanent support of the failing heart was possible. Since that time, the concept of MCS has continued to unfold, accompanied by advancing insights into cardiovascular physiology.
In the twentieth century, research into organ perfusion and preservation of the circulation was carried out by many investigators, including DeBakey (8), Lindbergh (9), and Gibbon (10), who laid the foundation for today’s MCS systems. In 1953, the clinical introduction of cardiopulmonary bypass ushered in the era of open heart surgery and highlighted the need for temporary support of the circulation (10). In a sense, the heart-lung machine may be considered the parent of mechanical assist devices. It fueled the imagination of many cardiovascular specialists and gave rise to 40 years of rapid development in assist-device technology.
A major breakthrough in MCS systems occurred in 1961, when Moulopoulos et al. (11) introduced the intraaortic balloon pump. This device was not used clinically until 6 years later, when Kantrowitz et al. (12) implanted it in a patient with cardiogenic shock. Since that time, it has been extremely valuable for treating potentially reversible left ventricular dysfunction. Intra-aortic balloon pump support remains the most common form of MCS.
Other early breakthroughs included the first use of a roller pump to assist the left ventricle, by Dennis et al. (13) in 1962. In addition, Spencer et al. (14) used an extracorporeal
roller pump to support patients in postcardiotomy cardiogenic shock. In 1963, DeBakey et al. (15,16,17) implanted a pulsatile pump in three patients who had undergone open heart surgery. This air-driven, tube-type pump, made of Dacron-reinforced Silastic with ball valves at the inflow and outflow ports, was the first ventricular assist device to be implanted clinically. Later, the same group used an extracorporeal ventricular assist device (VAD) in two patients who became long-term survivors.
roller pump to support patients in postcardiotomy cardiogenic shock. In 1963, DeBakey et al. (15,16,17) implanted a pulsatile pump in three patients who had undergone open heart surgery. This air-driven, tube-type pump, made of Dacron-reinforced Silastic with ball valves at the inflow and outflow ports, was the first ventricular assist device to be implanted clinically. Later, the same group used an extracorporeal ventricular assist device (VAD) in two patients who became long-term survivors.
In 1964, the National Institutes of Health (NIH) had established its Artificial Heart Program, whose goals included the development of both partial and total artificial hearts. At first, the research was multifaceted, using resources from medicine, the basic sciences, engineering, industry, and systems management. Throughout that decade, NIH programs emphasized the separate development of various components of circulatory support and replacement devices. This federal funding source continued the development of MCS for many years.
In 1969, Cooley (18) performed the first clinical bridge-to-transplant operation when he implanted a Liotta total artificial heart (TAH) in a 47-year-old terminally ill man. The pneumatically driven, diaphragm-type, dual-ventricular TAH was positioned orthotopically, replacing the native ventricles. It functioned well, sustaining the patient for 64 hours until a donor heart could be transplanted. Since that time, the TAH has undergone considerable evolution.
In 1982, William DeVries (19) performed the first of five TAH implantations intended to serve as permanent cardiac replacements. In these cases, the TAH was the Jarvik-7 model designed by Robert Jarvik. Although this pump was able to support the total circulation for prolonged periods, patients had to remain hospitalized and tethered to control consoles. In addition, the series was plagued by device-related complications, particularly infection and stroke, and only two of the patients survived for more than a year (20).
After 1970, the direction of government-sponsored research shifted. The NIH institute responsible for the Artificial Heart Program achieved bureau status and became the National Heart, Lung, and Blood Institute (NHLBI); MCS research was performed under the auspices of the Devices and Technology Branch of the Division of Heart and Vascular Diseases. In the mid-1970s, having performed extensive animal and in vitro tests of device safety and reliability, the NHLBI challenged physicians, engineers, and private companies to develop a fully implantable device for the long-term support of CHF patients. As a result, a wide range of VADs were introduced to support patients in postcardiotomy shock (21,22,23,24,25,26). Most of these devices were pneumatically actuated pulsatile pumps (27,28).
By 1978, the Texas Heart Institute was conducting clinical tests of an abdominally positioned left ventricular assist device (LVAD). That same year, Cooley and Norman (29) used this LVAD as a bridge to transplantation in a patient who had developed stone heart syndrome after undergoing valve replacement. The single-chambered, implantable device supported the patient’s circulation for 5 days until a donor heart became available. In 1986, we began testing a fully implantable LVAD, now called the HeartMate (Thoratec Corp., Pleasanton, CA), which was designed for long-term or permanent left ventricular support (30). The original, pneumatically powered version of this device began undergoing clinical testing in bridge-to-transplant patients in 1986 (31). Five years later, an electrically powered, more portable version of the HeartMate entered into clinical trials (32). Both versions of the HeartMate, pneumatic and electric, have completed clinical trials and were commercially approved by the U.S. Food and Drug Administration (FDA) in 1994 and 1998, respectively. The HeartMate electric LVAD was then commercially approved for destination therapy in 2003. Likewise, the Thoratec VAD (Thoratec Corp., Pleasanton, CA) and the Novacor N100 VAS (World Heart Corp., Oakland, CA) systems have also received commercial approval as a bridge to heart transplantation.
In the 1990s, a new generation of potentially more reliable and versatile MCS devices, the rotary pumps, were being developed. Today, axial-flow VADs such as the Jarvik 2000 Heart (Jarvik Heart, Inc., New York, NY) and HeartMate II (Thoratec Corp., Pleasanton, CA) are being tested clinically as bridges to transplant and show potential for use as permanent, long-term therapy. Others are now being applied in the catheterization laboratory and the operating room to cases of acute heart failure.
The clinical use of MCS systems is now widespread. Despite numerous design modifications and refinements, no ideal system has yet evolved. Nevertheless, current systems can salvage many patients with end-stage CHF, providing hope where none had previously existed.
Short-Term Circulatory Support
Settings in Which Short-Term Mechanical Circulatory Support May Be Useful
Short-term (up to 2-week) MCS is an important strategy for controlling acute, reversible heart failure presenting as cardiogenic shock. Often, this condition is caused by an acute myocardial infarction involving more than 40% of the left ventricular mass (33); in such cases, MCS can support the patient’s circulation until his or her condition improves enough for myocardial revascularization to be undertaken. Alternatively, cardiogenic shock may develop after open heart surgery; short-term mechanical support may allow the patient to be weaned from cardiopulmonary bypass and to regain adequate ventricular function. No matter what causes the cardiogenic shock, the chance of death is greater than 80% without MCS (34,35,36,37,38).
Of course, the most familiar form of MCS is cardiopulmonary bypass itself, which permits the heart to be stopped long enough for intricate repairs to be performed on it. Unfortunately, cardiopulmonary bypass and cardiac arrest can produce numerous adverse effects, including additional myocardial ischemic damage necessitating continued MCS (39,40). Today, acute coronary occlusions are usually managed with aggressive interventional procedures in the cardiac catheterization laboratory. In patients who have dissection, rupture, or reocclusion or who are otherwise at high risk, MCS is often used as a bridge to either balloon angioplasty or emergency coronary bypass. Other
possible indications for short-term MCS include acute myocarditis and cardiac dysfunction or labile pulmonary hypertension immediately after heart transplantation.
possible indications for short-term MCS include acute myocarditis and cardiac dysfunction or labile pulmonary hypertension immediately after heart transplantation.
Appropriate Devices for Short-Term Mechanical Circulatory Support
Intra-Aortic Balloon Pump
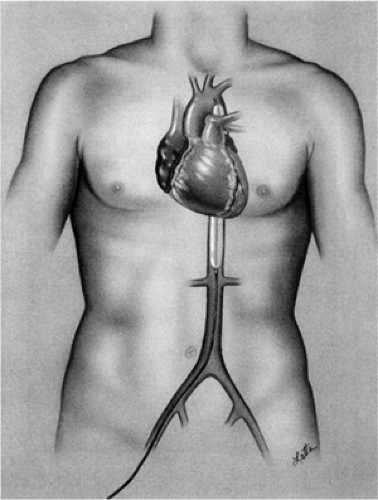
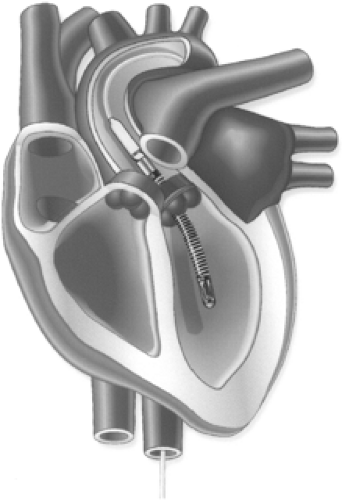
The world’s most widely used MCS system is the intra-aortic balloon pump (IABP), which was introduced during the 1960s by Moulopolous (11) and Kantrowitz (12). The polyurethane balloon is attached to the catheter, which is inserted percutaneously into the common femoral artery and is then advanced to the descending thoracic aorta (Fig. 45-1). Alternatively, if severe peripheral vascular disease is present the balloon may be inserted into the abdominal aorta via a retroperitoneal approach or, during open heart surgery, into the aortic arch via the subclavian artery.
The pump’s action depends on several unique but fairly simple physiological principles. At the start of diastole, the balloon inflates and forces blood distal and proximal to the catheter, which augments coronary perfusion by increasing the diastolic pressure in the aortic root. At the beginning of systole, the balloon deflates, producing a lower pressure within the aorta; blood is ejected from the left ventricle with less resistance, increasing the cardiac output by as much as 40% and decreasing the left ventricular stroke work and myocardial oxygen requirements (41). In this manner, the balloon supports the heart indirectly. Although designed to provide short-term MCS, the IABP has occasionally been used in heart transplant candidates for weeks at a time.
The device’s limitations include an inability to completely unload the left ventricle. In fact, the IABP will function only if the patient’s heart can produce at least some cardiac output and has a regular cardiac rhythm. Adequate IABP function depends on appropriate timing of the balloon cycle and is suboptimal in the presence of arrhythmias. Moreover, patients with this device cannot leave their hospital beds. In up to 43% of cases, the pump must be removed because of peripheral vascular hemorrhagic or thromboembolic complications, which may require surgical intervention (42,43). Despite the device’s life-saving capability, IABP recipients have a mortality that ranges from 7% to 86% (44).
ABIOMED BVS 5000 and AB 5000
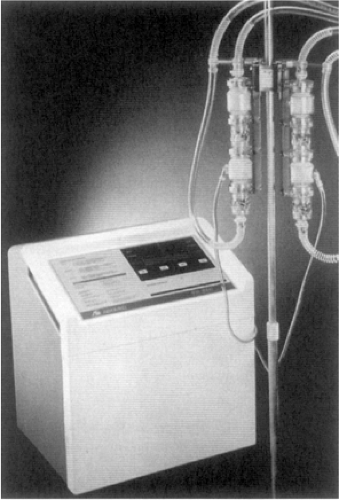
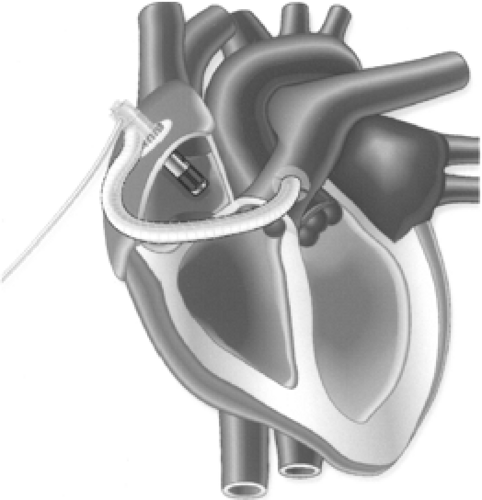
When short-term uni-ventricular or biventricular support is needed for patients with potentially reversible ventricular dysfunction, the ABIOMED BVS 5000 or AB 5000 (ABIOMED, Inc., Danvers, MA) devices may be used. These externally positioned systems, composed of one or two disposable blood pumps governed by a single console, provide pulsatile flow (Fig. 45-2) (45). Although the BVS 5000 system was introduced clinically in the late 1980s and the
AB 5000 in 2003, both systems are widely used today. Compared with the older technology, however, the AB 5000 provides a higher pump flow of approximately 1.5 L per minute and allows patient ambulation during use.
AB 5000 in 2003, both systems are widely used today. Compared with the older technology, however, the AB 5000 provides a higher pump flow of approximately 1.5 L per minute and allows patient ambulation during use.
The BVS 5000 is a dual-chambered, gravity-filled pump that provides approximately 5 L per minute of support (46). Within the pump, one-way flow is ensured by two trileaflet valves, one between the ventricular and atrial chambers and the other in the ventricular outflow tract. All blood-contacting surfaces within the pump are covered with a proprietary polyetherurethane (Angioflex) that is designed to be biocompatible and nonthrombogenic. Depending on the type of circulatory assistance needed, wire-reinforced 46 F inflow cannulas are inserted into the patient’s right or left atrium. At the distal end of each wire-reinforced outflow cannula is a 14-mm Angioflex-coated Dacron graft, which is connected to the aorta or pulmonary artery. To prevent infection, each cannula is covered with Dacron velour at its transcutaneous exit site.
The AB 5000 circulatory support system is used to support one or both sides of the heart. Each pneumatically driven, one-chambered pump utilizes the same valves and polyurethane material as the BVS 5000. For left-sided support, blood inflow from the left atrium or ventricle is returned to the thoracic aorta. For right-sided support, inflow from the right atrium returns to the pulmonary artery. Cannulation for the AB 5000 is similar to that for the BVS 5000.
A pneumatic, microprocessor-controlled drive console automatically controls the stroke volume of each pump and monitors filling by means of a driveline connected to the ventricular housing. The console adjusts the left and right blood pumps independently, and it automatically determines pump rates and systolic/diastolic intervals by sensing driveline airflow. Pumping is not synchronized with the heartbeat and the pump rate is increased or decreased depending on the rate at which the pump fills. During weaning, the flow rate is reduced by means of a control knob.
Patients supported by either ABIOMED device often require prolonged intensive care, including multiple intravenous medications, chest tube drainage, and invasive cardiovascular monitoring. Many will have undergone extensive cardiac surgery with cardiopulmonary bypass (47). Because use of either system entails a risk of thromboembolism, patients must take anticoagulants (48). Bleeding and reoperation are the most frequently reported complications. In most cases, ambulation and physical rehabilitation are very difficult. Ambulation with the AB 5000 pump is possible because the pump is vacuum-filled; ambulation with the gravity-filled BVS 5000 pump is not likely. Patients who cannot be weaned from support are considered candidates for implantation of a long-term MCS device.
Over 6,000 patients have been supported with the BVS 5000 and 88 patients have been supported with the AB 5000. Indications for BVS 5000 use have included postcardiotomy cardiogenic shock in 63% of cases, acute myocardial infarction (AMI) cardiogenic shock in 9%, failed heart transplant in 8%, myocarditis in 2%, and other indications in 18%. A similar trend has been noted for the AB 5000, although it has also been used to provide right heart support after LVAD implantation.
Medtronic Perfusion Systems Pump
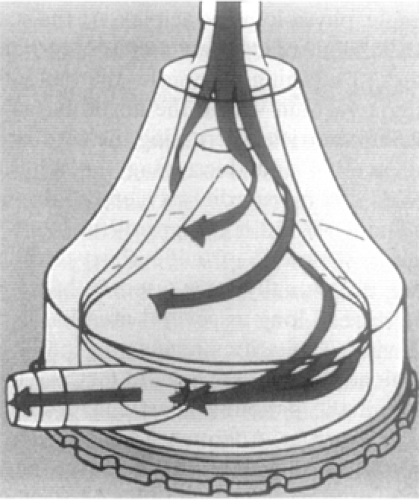
Another option often used for short-term uni-ventricular or biventricular MCS is the Medtronic Perfusion Systems Bio-Pump (Medtronic, Inc., Minneapolis, MN; formerly miomedicus pump), an extracorporeal centrifugal device available in two disposable models: an 80-mL model for adults (maximal flow, 10 L minute) and a 48-mL model for children. In this device, rotating acrylic cones generate continuous flow according to the constrained vortex principle (Fig. 45-3). The pump is magnetically coupled to an external motor and console where impeller speed is manually adjusted.
Clinicians have acquired considerable experience with the Bio-Pump because it is primarily used for cardiopulmonary bypass and is available in the majority of centers that perform cardiac surgery. Its advantages include simplicity, versatility, and cost-effectiveness. Limitations include the need for specialized personnel to supervise the system and the need for anticoagulation. Moreover, durability or thrombosis problems preclude use of the Bio-Pump for more than 5 days, although this limit is occasionally exceeded. In any case, the pump component should be replaced every 48 to 96 hours. Despite heparin bonding, the pump circuit is susceptible to thrombus formation; systemic heparinization is often necessary, which can intensify bleeding complications (49). The externalized cannulas are also potential sites of infection.
Beyond its cardiopulmonary bypass applications, the Medtronic Perfusion Systems pump is mainly used to provide temporary support in patients with postcardiotomy heart failure (50,51,52). It has also been used to support children in cardiogenic shock following repair of congenital heart defects (53) and to provide temporary right heart support following heart transplantation or LVAD implantation (54,55). Although more than half of patients supported with the Medtronic Perfusion Systems pump can be weaned from support, the hospital discharge rate is only 22% (56). Frequent and severe complications due to comorbidities are common during support in this population.
Tandem Heart
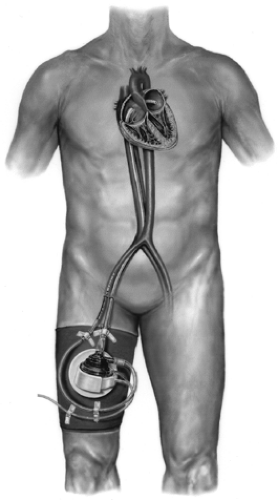
The Tandem Heart percutaneous VAD (Cardiac Assist, Inc., Pittsburgh, PA) is intended for short-term use for up to 14 days in cases of acute heart failure (Fig. 45-4). The Tandem Heart is very small, with a priming volume of only 7 mL, but can produce continuous flows of up to 3.5 L per minute. A heparinized bearing lubrication system protects the impeller from wear and helps to prevent thrombosis within the pump, thus minimizing the need for anticoagulants. The device is inserted percutaneously through a femoral vein and advanced across the intra-atrial septum into the left atrium. Blood is withdrawn from the left atrium through a 21-F transseptal cannula and pumped to one or both femoral arteries through 15 or 17 F outflow cannulas.
The Tandem Heart has been used to provide support during high-risk percutaneous coronary intervention (PCI), cardiogenic shock following AMI, and postcardiotomy heart failure (57). In most cases of high-risk PCI, the device is removed at the end of the procedure or within a few hours if the postoperative course is uncomplicated. In cases of cardiogenic shock or postcardiotomy heart failure, it is used to support patients until sufficient cardiac recovery occurs or, barring that, until more definitive longterm therapy can be considered.
At present, the clinical experience with the Tandem Heart is small (57,58). At our institution, we have applied it with some success in seven cases of high-risk PCI, six cases of bridging to implantation of a long-term VAD, eight cases of cardiogenic shock, and three cases of support during cardiac surgical procedures.
Investigational Devices for Short-Term Mechanical Circulatory Support
Two promising short-term VAD systems are undergoing clinical trials: the Impella Recover (Impella CardioSystems AG, Aachen, Germany) and Levitronix CentriMag (Levitronix LLC, Waltham, MA). Both have made it through FDA phase I studies and have entered phase II pivotal trials. These new systems are intended to support patients for up to 2 weeks while myocardial function recovers or, in the case of no recovery, until implantation of a long-term VAD. The most frequent indications for their use are AMI and failure to wean from cardiopulmonary bypass.
Impella Recover
The Impella Recover is a miniature catheter-mounted pump that can provide either left or right heart support at flows of 5 to 6 L per minute. The device can be threaded percutaneously through a femoral artery into the ventricle for support during acute heart failure or inserted directly into the ventricle through a sternotomy for support during postcardiotomy heart failure. The Impella Recover has been used extensively in Europe (59,60) and has only recently been introduced in the United States for clinical trials.
The device has been used for circulatory support in more than 350 patients worldwide, including left heart support in the majority, right heart support in 23, and biventricular support in 11. Left heart support is provided by the Recover LD version (Fig. 45-5) and right heart support by the Recover RD (Fig. 45-6). Indications have included support during high-risk percutaneous transluminal coronary angioplasty, support during cardiogenic shock following acute myocardial infarction, and failure to wean from cardiopulmonary bypass. On several occasions, this system has been used as a short-term bridge to heart transplant or for support during off-pump coronary artery bypass procedures.
Levitronix CentriMag
The Levitronix CentriMag system is designed to provide circulatory support for up to 14 days to patients suffering from severe, acute, but potentially reversible cardiac failure (61). The system, which can be readily adapted to available cannulas and tubing, consists of a blood pump, motor, and drive console (Fig. 45-7). Within the pump’s housing, which contains no seals or bearings, a magnetically levitated impeller rotates at speeds of up to 5,500 rpm and can produce a centrifugal flow of 10 L per minute. This contact-free pumping mechanism is intended to enhance biocompatibility and reliability by eliminating the friction and heat that can contribute to hemolysis, thrombosis, and wear.
In Europe, the CentriMag is approved for use as an extracorporeal MCS device for up to 14 days in patients in severe, potentially reversible ventricular failure. In the United States, it is still considered an investigational device but has entered a clinical trial to evaluate its safety and effectiveness in the treatment of postcardiotomy cardiogenic shock under an FDA exemption.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree