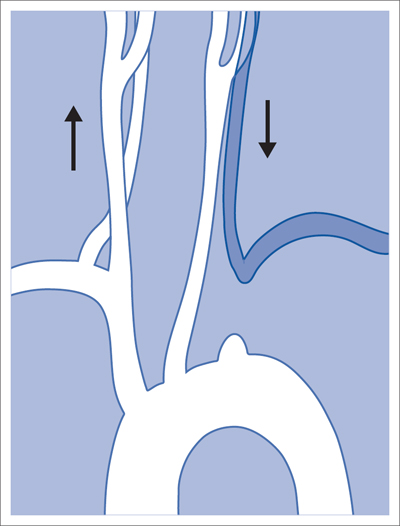
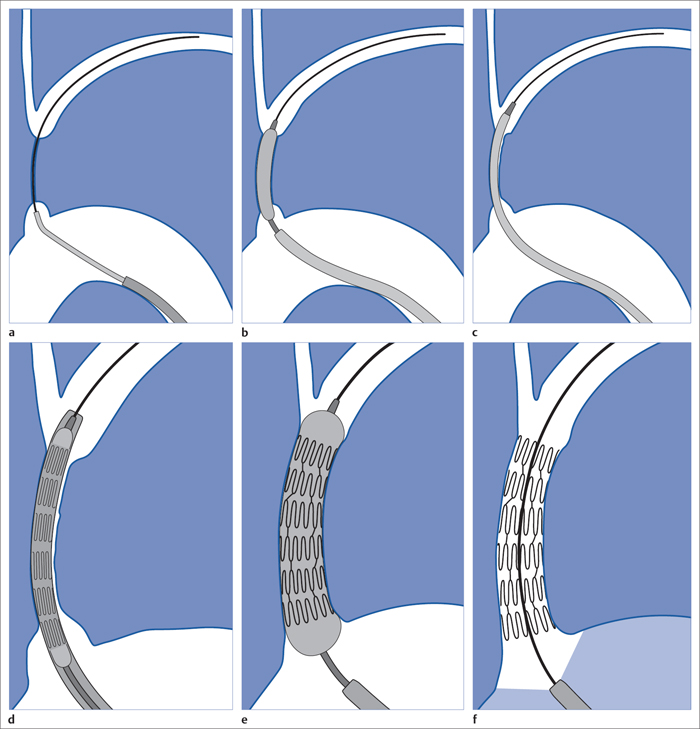
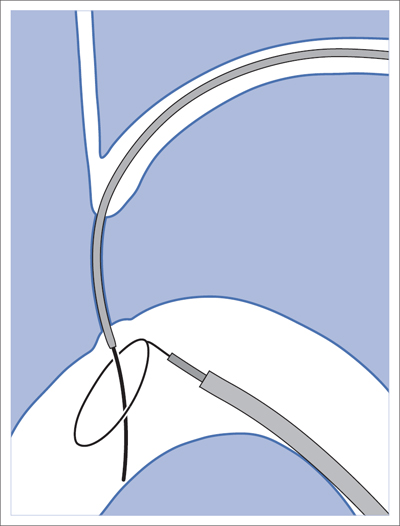
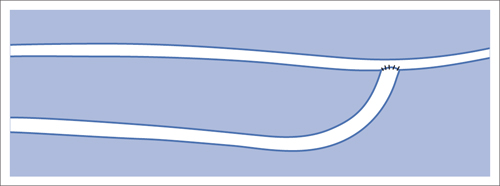
6 Upper Extremities There is only one intervention in the branches of the aortic arch (supra-aortic branches) that is largely free of the risk of a cerebral insult, that is not particularly demanding from a technical standpoint, and that has been performed since the beginning of interventional radiology: percutaneous transluminal angioplasty (PTA) of the left subclavian artery. A stenosis develops relatively often between the origin of the subclavian artery on the aortic arch and the origin of the left vertebral artery. This stenosis can progress to an occlusion in the further course of the disorder. This leads to reduced blood pressure and, in advanced cases, weakness in the left arm. The only major collateral that can mitigate the problem for the left arm is the left vertebral artery. The posterior portion of the cerebral circulatory system is supplied by two arteries that join together to form the trunk of the basilar artery. Stenosis of the left subclavian causes blood pressure to decrease in the left vertebral and brachial arteries until blood flow in the left vertebral artery reverses. Where the condition has progressed to complete occlusion of the subclavian artery, the left arm is supplied nearly exclusively via the vertebral artery. The simple flow reversal, which can be well visualized angiographically (see Chapter 3, Angiography of the Upper Extremities, p. 88) is referred to as subclavian steal or subclavian steal phenomenon (Fig. 6.1). One refers to a subclavian steal syndrome when this tapping of the vertebral artery by the left arm triggers symptoms of cerebral ischemia. The typical symptom of vertigo occurs when physical exertion increases the blood required by the left arm. Intervention is regarded as indicated where the typical symptoms of the subclavian steal syndrome are present, or at least the weakness of the arm represents a significant impairment, and when a stenosis of at least 70 to 80% in the left subclavian artery suggests that the intervention will eliminate the symptoms. The technical success rate for stenosis is close to 100%. In the presence of an occlusion, catheterization may fail. The flow reversal invariably present in the vertebral artery largely excludes any cerebral complications from the intervention. Because the subclavian artery is an elastic vessel, the rate of recurrence is relatively low at 5 to 7% (Kedhi et al 2008). Fig. 6.1 Occlusion of the left subclavian artery at a typical location with flow reversal in the left vertebral artery. Early phase (arrow up); late phase (arrow down). A thoracic aortogram is obtained via a transfemoral approach to visualize findings. Then one usually advances an 80 to 90 cm sheath up to the origin of the left subclavian artery. In most cases a vertebral artery catheter (straight with a slight bend close to the tip) is suitable for catheterizing the subclavian artery. Place the tip of this catheter into the origin of the subclavian artery and then advance a straight glide wire through this catheter into the stenosis or occlusion (Fig. 6.2). If the wire is not able to pass through an occlusion, attempt to find a better approach by changing the position of the catheter tip. One can also try a slightly curved glide wire or an 0.018 in. guidewire with a short flexible tip (preferably one with a core of nitinol, e.g., the Terumo Glide Wire Advantage, Terumo Interventional Systems, Somerset, NJ, USA). Fig. 6.2 Recanalization of an occlusion of the left subclavian artery. a Advance wire. b Preliminary dilation. c Advance sheath into occlusion. d Place balloon-expandable stent within sheath in the occlusion. e Withdraw sheath, dilate. f Evaluate results with angiography. Access via the left brachial artery is an alternative. (In the absence of a palpable arterial pulse, a Doppler probe should be used to locate the artery for the puncture.) Many times it is possible to pass a wire from the arm through an occlusion that cannot be penetrated from the aortic arch. One can capture the long wire with a snare, pull it out through the sheath in the common femoral artery, and then use it as a track for advancing the balloon catheter (Fig. 6.3). Naturally, the entire intervention may be performed via the brachial artery. However, then it is not as easy to place a stent with great precision. In the usual case of catheterization via the groin only, advance the wire into the brachial artery prior to PTA so that there is a sufficiently reliable track for overcoming resistance. Because of the more favorable results, the method generally preferred today is to treat not only occlusion but also stenosis by placing a stent. Stenoses are more often encountered close to the origin of the vessel, where precise placement is crucial. For this reason balloon-expandable stents are preferable. To select the correct stent one must measure both the width of the vessel and the length of the lesion. The stent should not cover the origins of the vertebral and internal thoracic arteries. In an ostial stenosis the stent should project 1 to 2 mm into the aorta as in the renal arteries. In a stenosis distal to the origin of the vertebral artery (which does not lead to a subclavian steal syndrome), a self-expanding stent is of course preferable. Stenoses in the right subclavian artery are encountered much less frequently than in the left subclavian artery. Endovascular management of these lesions is riskier and far more challenging. The same applies to stenoses in the brachiocephalic trunk and left carotid artery. Except in the hands, arteriosclerotic stenoses and occlusions occur relatively rarely in the arteries of the arm. Primarily in elderly women one will occasionally encounter chronic occlusions in the axillary artery and upper arm in the setting of giant cell arteritis. Thickening of the vascular wall is a typical ultrasound finding. These disorders are treated with immunosuppressants. Careful dilation can bring relief of symptoms in certain cases when the inflammation is well controlled. There are two common types of arteriovenous dialysis shunts: the Cimino-Brescia arteriovenous fistula and a polytetrafluoroethylene (PTFE) graft interposed between an artery and vein. The arteriovenous fistula is usually created between the radial artery and the cephalic vein at a distal location in the nondominant arm. It is usually created as a side-to-end anastomosis (Fig. 6.4). This shunt must “mature” over a period of 3 to 4 months (i.e., the vein must increase significantly in width due to the increased pressure and flow volume and its wall must become thicker before it can be used for dialysis). The interposed PTFE graft (Fig. 6.5) has the necessary width right from the start and can therefore be used within 2 weeks of implantation. Fig. 6.5 Two options for placing polytetrafluoroethylene graft in the forearm. a Interposed graft between distal radial artery and vein in the proximal forearm. b Interposed graft between proximal radial artery and vein in the proximal forearm (loop). The average duration of initial patency for arteriovenous fistulas is 3 years, for PTFE grafts only about 1 year. Interventional measures or surgical corrections can extend these periods (secondary patency) to 7 and 2 years, respectively (Calabrese and Yasin 2008). This shows the importance of the task entrusted to the interventional radiologist along with the surgeon. Repeated interventions can double the useful life of a dialysis shunt!
Stenosis or Occlusion of the Left Subclavian Artery
Performing the Procedure
Access via the Brachial Artery
Stenoses and Occlusions in Other Branches of the Aortic Arch
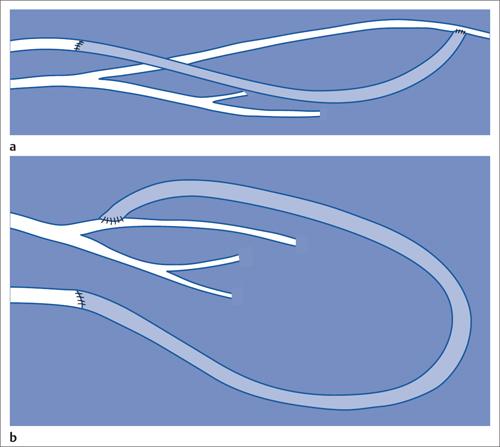
Dialysis Shunt
General

![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree