Chapter 22 During fetal life, the ductus carries the majority of the outflow of the right ventricle (RV) to the descending aorta. Spontaneous functional closure of the ductus arteriosus usually occurs after delivery by approximately 15 hours of age,1 with permanent closure occurring within several weeks. Persistent PDA is estimated to occur in approximately 0.8/1000 live births,2 exclusive of silent PDA. The prevalence of PDAs decreases with age, but the true prevalence in the adult population is unknown because many cases of hemodynamically insignificant PDA have no signs or symptoms and patients remain undiagnosed. Silent PDAs (those in whom no pathologic murmur is audible) are estimated to occur in up to 0.5% of patients referred for echocardiographic evaluation of an innocent murmur3; however, the clinical significance of these is controversial. The clinical manifestations of a PDA are dependent upon the direction and degree of shunting across the PDA. Factors that control the direction and magnitude of the shunt across the PDA include the diameter and length of the ductus arteriosus, the pressure difference between the aorta and pulmonary artery (PA), and the systemic and pulmonary vascular resistances.4 If the shunt across the PDA is small, no signs or symptoms may be present, or a murmur heard during physical examination might be the only abnormality. Patients are typically asymptomatic. The murmur may be a soft systolic murmur best heard at the left upper sternal border or a continuous murmur. The murmur is often harsh and may have a “machinery” quality. The presence of the murmur does not necessarily imply that the PDA is hemodynamically significant, because the murmur most likely results from the jet from the PDA striking the anterior aspect of the PA.5 Likewise the absence of a murmur does not imply that the PDA is hemodynamically insignificant, because left heart dilation has been found even in the absence of a murmur.6 Chest x-ray findings may be normal with a small PDA, as may the electrocardiograph (ECG). Echocardiography in a parasternal short-axis view is diagnostic with color Doppler demonstrating the left-to-right shunt The only potential indication to close a small hemodynamically insignificant PDA is prevention of endarteritis. This is a controversial topic. A recent review on this subject by Fortescue et al8 estimated that the risk of endarteritis associated with this type of a PDA is between 0.01% and 0.001% per year. These authors also reviewed 10 published papers on transcatheter PDA closure as well as their own institutional experience. They reported a major overall adverse event rate of 1.5%, with a minor adverse event rate of 8%. Their conclusion was that “there is no evidence to support a superior risk/benefit balance for routine closure of the very small hemodynamically insignificant PDA, and accordingly, it is difficult to justify closure of such defects simply to reduce the risk of infective endocarditis and its complications.” According to 2008 guidelines from the American College of Cardiology/American Heart Association (ACC/AHA),9 routine follow-up examination every 3 to 5 years is recommended for patients with a small PDA and no evidence of left heart enlargement (Class I recommendation; Box 22–1). PDAs of 2 mm or smaller at the narrowest portion are suitable for coil embolization. Coils are available in a variety of shapes and sizes (Figure 22–1). PDAs larger than 2 mm in diameter may be closed with coils, but the embolization risk is higher, and multiple coils and specialized techniques may be required. Therefore for larger PDAs, device closure using the Amplatzer ductal occluder (ADO; St. Jude Medical, St. Paul, Minn.) is preferred. Ductal morphology is important, in addition to size of the ductus. The Krichenko classification is generally used for PDA morphology (Figure 22–2).10 Figure 22–1 Embolization coils and delivery device. Figure 22–2 Krichenko classification. 1. Establish femoral venous and arterial access. Perform a right and left heart catheterization to assess the degree of shunt and pulmonary pressures and resistance. 2. Perform an aortogram. The straight lateral projection usually profiles the PDA quite well. Steep right anterior oblique (≈45 degrees) can also be used to elongate the ductus. The catheter should be positioned in the descending aorta near the left subclavian artery. 3. Assess the morphology of the PDA from the angiogram (see Figure 22–2). Measure the narrowest portion of the PDA. If the PDA is 2 mm or smaller in diameter, coil embolization may be performed. If the diameter is larger than 2 mm, device deployment is easiest. 4. Using an angled catheter such as a Judkins right coronary catheter, a soft-tipped 0.018-inch or 0.035-inch floppy wire is advanced retrograde across the ductus from the aorta. The catheter is then advanced over the wire and into the MPA, and the wire is subsequently removed. 5. A coil that has a diameter of at least twice the narrowest diameter of the ductus is chosen. The length of the coil should be such that approximately 4 loops of coil will form. This allows one loop to be placed in the MPA side and the remaining loops within the aortic ampulla. A quick way to determine this is to divide the length of the coil in millimeters (mm) by (π (≈3) × the coil diameter loop in mm). For example, if a 0.035-5-5 coil is chosen, the length is 5 cm (50 mm). 50 is divided by 15 (3 × 5), which yields approximately 3.3 loops of coil. Thicker gauge coils such as 0.035-inch, 0.038-inch, or even 0.052-inch are generally preferred. The thicker coils have more radial strength and are less likely to embolize. 6. The coil is loaded into the delivery catheter and advanced by using the soft end of a straight wire. For example, if a 4F delivery catheter is chosen with an internal dimension of 0.038-inch, and a 0.038-inch coil is used, a 0.035-inch or 0.038-inch wire may be used to advance the coil. 7. One half to one loop of coil is extruded into the MPA, and the catheter and coil are pulled back as a unit toward the aorta until the coil loop is positioned at the MPA end of the ductus. This is best appreciated on the straight lateral projection. For most PDAs this is at the anterior aspect of the tracheal air column. 8. Once the coil is positioned at the MPA end of the PDA, the delivery catheter is pulled into the aorta. The push wire can be used to advance coil loops into the aortic ampulla as the catheter is pulled back. The goal is to deliver one half to one loop on the PA end of the PDA and 3 to 4 loops in the aortic ampulla (Figure 22–3). This method of coil deployment does not allow proximal or distal control of the coil loop; therefore coil maldeployment or embolization are risks of the procedure. In order to have more controlled delivery of the coil, multiple techniques have been developed, including snaring the coil in the MPA,11 the bioptome-assisted technique,12 the balloon-occlusion technique,13 and a combination of the snare and bioptome-assisted techniques.14 For a full description of these techniques, please consult the referenced articles. In addition to these methods, controlled release or detachable coils such as the Flipper coil (see Figure 22–1, B; Cook Medical, Bloomington, Ind.) are available.15,16 These coils are quite useful because they allow repositioning and decrease the risk of embolization. The ADO (Figure 22–4) is the most commonly used device to close moderate to large PDAs since approval by the U.S. Food and Drug Administration in 2003. The ADO has an aortic retention disk with an attached slightly conical-shaped body. The body tapers in diameter away from the aortic retention disk. The device is intended for implantation from a transvenous approach and is deployed through a 5F to 7F long sheath. Figure 22–4 Amplatzer occlusion devices. 1. Similar to coil delivery, an aortic angiogram is performed (Figure 22–5). The device is chosen so that the smaller end of the device is 2 mm larger than the narrowest diameter of the PDA. If the PDA is tubular in shape (Krichenko type C, Figure 22–6, A), an Amplatzer vascular plug II can be used, and also should be sized 2 mm bigger than the PDA diameter (Figure 22–6, B). Figure 22–5 Lateral angiogram demonstrating type A ductus suitable for closure using the Amplatzer duct occluder. Figure 22–6 A, Krichenko type C ductus (tubular). This type of ductus is difficult to occlude with coils or the duct occluder and is most suitable for the Amplatzer vascular plug II. B, Lateral angiogram after closure of the tubular ductus demonstrating appropriate positioning and complete ductal closure. 2. After a device is chosen, the PDA is crossed from the PA end and an appropriately sized TorqVue delivery sheath (St. Jude Medical, Plymouth, Minn.) is placed over a wire across the PDA. In many adults it is very difficult or impossible to cross the PDA antegrade from the MPA because the ductus tends to be inserted in the roof of the PA. In this situation, the ductus is crossed retrograde from the aorta with a wire, and a snare is placed transvenously into the MPA. The wire is snared in the MPA, and then the wire is pulled into the descending aorta as the snare and catheter are advanced into the descending aorta. The snare is released and removed from the body, leaving the snare catheter in the descending aorta. A 0.035-inch exchange wire is advanced through the snare catheter into the aorta, and the snare catheter is then removed. The TorqVue sheath is advanced over the wire and into the descending aorta, the wire and dilator are removed, and the sheath is flushed with saline. 3. The ADO is threaded onto the delivery cable, loaded into the delivery system, and advanced through the long sheath. The aortic retention disk is delivered into the aorta. Attention should be made not to perforate the descending aorta while doing so. The sheath tip should not be against the back wall of the aorta during advancement of the device. 4. Once the retention disk is delivered, the whole delivery system is pulled back until the disk is well seated in the aortic ampulla, and then the remainder of the device is deployed. The airway often serves as a useful landmark for the PDA on the lateral view and positioning of the device. 5. An aortic angiogram is performed before releasing the device. This will confirm device position and assess for residual shunts. Once the device is in satisfactory position, it can be released from the delivery system (Figure 22–7). A recent review of transcatheter PDA closure was reported from Boston Children’s Hospital.8 The authors included their own institutional experience for a total of 1808 patients. Complete closure was reported in 94% of patients with follow-up periods ranging from 1 day to 1 year (86% to 100%). There were a total of 18 major adverse events (1.5%) and 169 minor adverse events (8%). Major adverse events occurred in 10 patients in whom the device/coil embolized and was not retrieved and in six patients in whom an additional catheter or surgery was required for repair. In addition there was one patient death that was not related to the procedure and one case of endocarditis. Minor adverse events included 70 patients in whom a device embolized and was retrieved at the same procedure. For the overall series, device embolization occurred in 1.4% to 11.6% of patients. Other minor adverse events included mild aortic or left pulmonary artery (LPA) narrowing, arrhythmias, hemolysis, pulse loss, and the need for a blood transfusion. Coronary artery fistulas (CAFs) are a rare congenital or acquired abnormality in which a coronary artery branch terminates in a low-pressure vessel or chamber (Figure 22–8 and Video 22–2, Video 22–2A, Video 22–2B, Video 22–2C). Figure 22–8 A, Angiogram of a large left coronary fistula to the right ventricle (RV) in an infant. B, Echocardiogram with color Doppler in modified apical five-chamber view. C, RV inflow view demonstrating the fistula opening under the tricuspid valve (TV). CAFs were first described by Wilhelm Krause in 1865 in an otherwise healthy 53-year-old man who was ultimately found to have a large ramus intermedius-to-pulmonary artery fistula,20 and successful surgical closure was first described by Biorck and Crafoord in 1947.21 Interestingly, the first transcatheter closure was performed in 1981 by Reidy and colleagues22 after successful angioplasty of a severe left anterior descending artery (LAD) lesion. The operators then directed a detachable balloon to the distal circumflex to occlude the fistula to the bronchial artery. Follow-up angiography approximately 7 months later demonstrated persistent occlusion of the CAF as well as patency of the circumflex and LAD.22 Since those historic milestones, the incidence of congenital CAFs in the general population has been estimated at 0.002%.23 A systematic review of approximately 125,000 angiograms performed at the Cleveland Clinic during the years of 1960 to 1988 revealed an incidence of 0.12% “small coronary fistulas” and 0.05% “multiple or large-sized coronary fistulas”; however, patients with congenital heart disease or other coronary anomalies were excluded from analysis.24 In contrast, acquired coronary fistulas are even rarer and can occur as a result of complications of cardiac surgery,25 endomyocardial biopsy,26,27 or coronary angioplasty.28 Traumatic CAFs result most commonly from penetrating chest trauma involving the coronary artery and have a more acute natural history than coronary fistulas resulting from other etiologies.29,30 Coronary fistulas can also be associated with complex congenital heart disease, specifically single ventricle abnormalities such as pulmonary atresia with intact ventricular septum31 or hypoplastic left heart syndrome.32–34 These diffuse fistulas, often referred to as sinusoids, are associated with poor outcome caused by inadequate myocardial perfusion and additional volume load. The diffuse nature of these fistulas are also generally less amenable to closure or ligation The pathophysiology and clinical presentation of a CAF results from the absence of an intervening capillary bed. Symptoms are largely dependent on the size of the vessel and the chamber with which it communicates. For example, large coronary fistulas that drain into right-sided chambers and systemic veins can cause a left-to-right shunt and volume overload with heart failure symptoms (Table 22–1). Alternatively, the high-flow run-off can produce steal from the myocardial capillary bed, producing myocardial ischemia with anginal symptoms such as chest pain or dyspnea on exertion. Myocardial infarction can result because the large caliber fistula can serve as a nidus for thrombus formation (especially in aneurysmal vessels), which can either propagate retrograde into a branch coronary artery or embolize distally. Cerebral infarction has been reported as a result of thrombus embolization from a coronary fistula to the RA across a patent foramen ovale.35 TABLE 22–1 Definition of Size of Coronary Artery Fistulas, Pathophysiology, and ACC/AHA Guidelines for Management9 Smaller fistulas are often asymptomatic and may be monitored; a 10-year follow-up study in one series demonstrated that these patients are unlikely to develop symptoms or enlargement of fistulas.36 Finally, endocarditis may occur in up to 3% of medium and large coronary fistulas.37 Whereas small coronary fistulas are silent on physical examination, large coronary fistulas may result in both continuous systolic and diastolic murmurs and can often been identified on transthoracic echocardiography (see Figure 22–8, B and C, and Video 22–2, Video 22–2A, Video 22–2B, Video 22–2C B and C). Aneurysm formation of the coronary fistula up to 4 or 5 cm in diameter has been reported,19,39 with a rate of up to 26% in one series.40 Aneurysm formation in coronary fistulas may be related to an underlying inflammatory process.35 Increased shear stress caused by increased flow as well as turbulence may predispose the vessel to mural thinning as well as thrombus formation. Coronary fistula aneurysm rupture can occur, resulting in tearing chest pain (with or without radiation to the back), syncope, and, ultimately, tamponade.41,42 Rarely, mitral regurgitation can be seen as a result of a left circumflex to coronary sinus fistula.43 Guidelines for the treatment of coronary fistulas were published by the ACC and the AHA in 20089 (see Table 22–1). Although this chapter focuses on transcatheter closure, surgical treatment of coronary fistulas continues to play an important role, especially in patients with other cardiovascular issues requiring surgical intervention. Surgical closure of large coronary fistulas has been safe and feasible. Approximately half of surgical procedures published were performed off cardiopulmonary bypass when taking an extracardiac approach, whereas intracardiac repair required cardiopulmonary bypass, but the rate of residual shunt has been reported to be lower. Regardless of approach, overall mortality has been about 1%.18,44 Older adults were more likely to have complications such as arrhythmia (2% to 10%), myocardial ischemia (14%), infarction (2%), or postpericardiotomy syndrome (10%).45,46 In addition, follow-up angiography in one series demonstrated that 19% of the parent coronary arteries had either thrombosed or become threadlike, and 19% had residual fistulous flow.46 Two cases have resulted in aneurysmal dilation of the LAD with thrombus formation, myocardial infarction, or left ventricular aneurysm formation, and one patient has gone on to develop mitral regurgitation.47 Finally, very late (up to 10 years) thrombus formation with myocardial infarction can occur after surgical closure.48,49 Taken together, as with all cardiac surgery, patient-specific risks as well as procedural risks must be balanced against the risks of the coronary fistula. As such, the transcatheter approach to closure of coronary fistulas has become an appealing alternative. Although data are limited regarding outcomes related to patient factors in transcatheter closure,50 factors that play a role in coronary interventional outcomes may be extrapolated and include renal insufficiency, congestive heart failure, diabetes mellitus, and peripheral arterial disease (especially involving the aortoiliofemoral system). Certainly, atherosclerotic coronary artery disease may interfere with or complicate transcatheter closure of the coronary fistula. The approach to closure of a coronary fistula is dependent on the origin and termination of the fistula (Figure 22–10).51 In the distal type of coronary fistula, the fistula is located at the distal end of the coronary artery, inducing dilation of the entire coronary vessel. Intuitively, closure should be directed toward the distal fistulous segment. In contrast, the proximal type of coronary fistula branches early from the main coronary artery, creating a large conduit vessel that terminates in the low-pressure chamber or vessel. Latson and coworkers have recommended closing this type of fistula at both the distal termination site as well as the near the proximal take-off of the fistula in order to prevent the formation of a large blind pouch and attendant nidus for thrombus with possible embolic complications from both the coronary end and the cardiac-chamber end.17 Figure 22–10 Classification of coronary fistulas.
Unwanted Vascular Communications
Patent Ductus Arteriosus, Coronary Fistulas, Pulmonary Arteriovenous Malformations, and Aortopulmonary Collaterals
22.1 Patent Ductus Arteriosus
Background
Diagnosis and Clinical Presentation
![]() (Video 22–1). Echocardiography is also used to assess the hemodynamic significance of the PDA by measuring the size of the LA and LV and to assess for signs of pulmonary hypertension. Other testing such as a computed tomography (CT) scan or magnetic resonance imaging (MRI) are usually not necessary to diagnose a PDA7 (although they may be helpful in situations in which the echocardiographic assessment is suboptimal) and often leads to overestimation of the size of the PDA.
(Video 22–1). Echocardiography is also used to assess the hemodynamic significance of the PDA by measuring the size of the LA and LV and to assess for signs of pulmonary hypertension. Other testing such as a computed tomography (CT) scan or magnetic resonance imaging (MRI) are usually not necessary to diagnose a PDA7 (although they may be helpful in situations in which the echocardiographic assessment is suboptimal) and often leads to overestimation of the size of the PDA.
Management
Procedural Technique
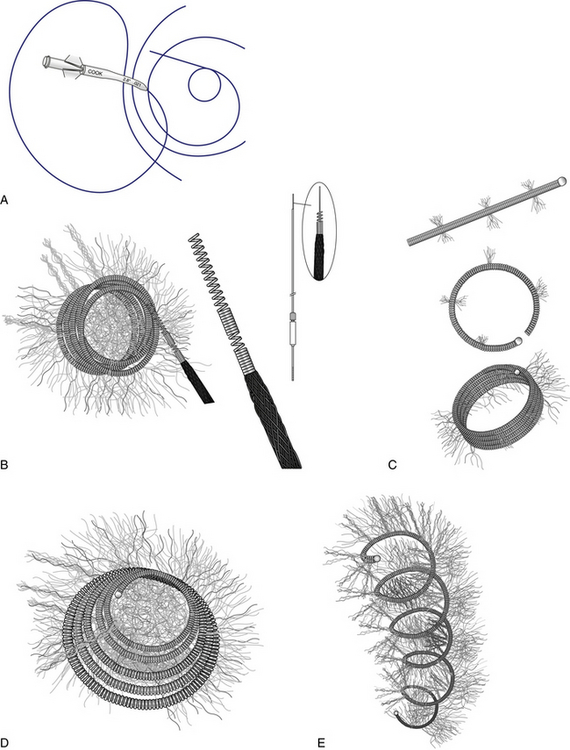
A, Cantata microcatheter (2.5F or 2.8F available). B, Flipper MREye 0.035 detachable coil and delivery system. C, Hilal 0.018-inch higher radial force microcoil. D, Tornado 0.018-inch and 0.035-inch soft coil. E, MREye Inconel 0.035-inch higher radial force coil. (Permission for use granted by Cook Medical Incorporated, Bloomington, Ind.)
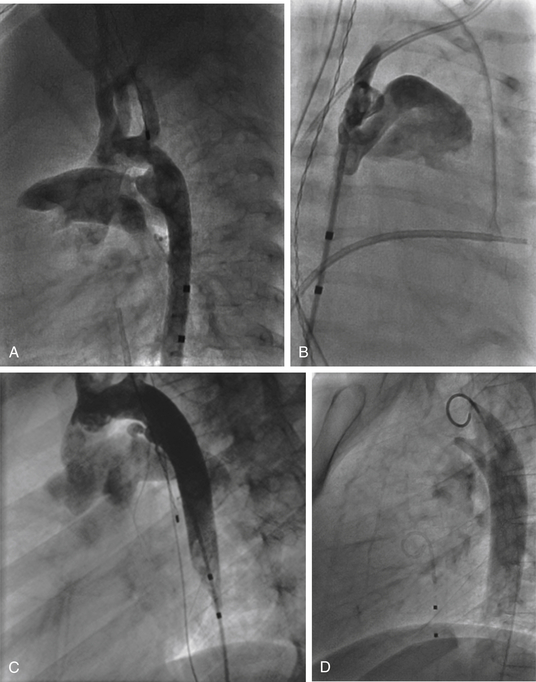
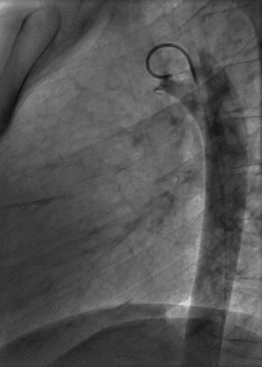
A, Type A ductus with narrowest insertion near the main pulmonary artery and short, conical-shaped ductus with well-developed aortic ampulla. A type B ductus is not shown; this patent ductus arteriosus (PDA) is short with the narrowest site at the aortic end. B, Right anterior oblique view of type C ductus, which is tubular and has no constriction. C, Type D PDA with multiple constrictions. D, Type E PDA is an elongated conical ductus with the narrowest insertion anterior to the anterior border of the airway on a lateral projection.
Coil Occlusion
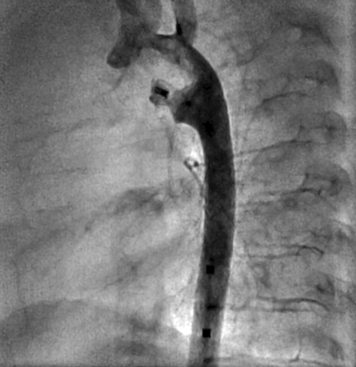
Device Occlusion
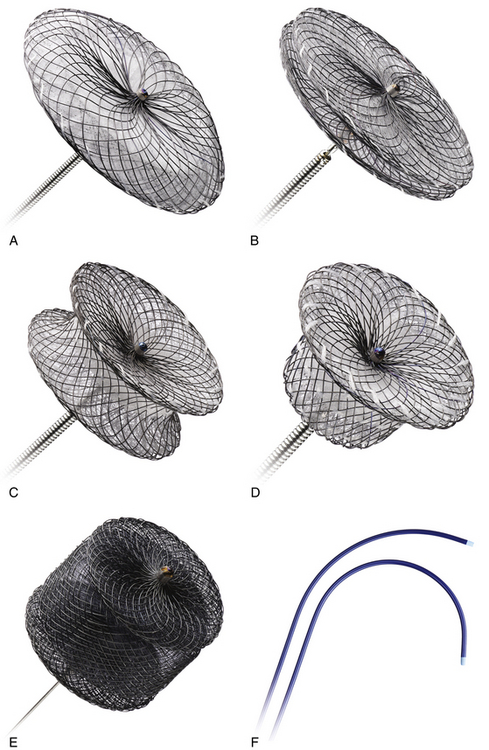
A, Amplatzer septal occluder. B, Amplatzer multifenestrated septal occluder (Cribriform Amplatzer duct occluder). C, Amplatzer muscular ventral septal defect occluder. D, Amplatzer duct occluder. E, Amplatzer Vascular Plug II. F, Amplatzer TorqVue 45-degree and 180-degree delivery systems. (Reprinted with permission of St. Jude Medical, St. Paul, Minn., 2012. All rights reserved.)
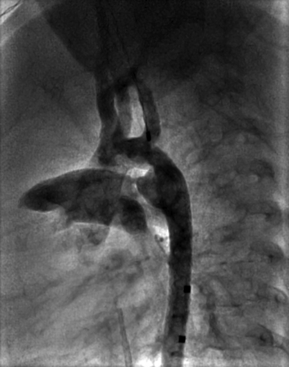
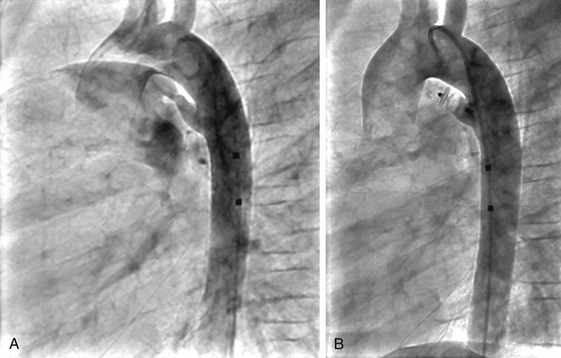
Outcome and Complications
22.2 Coronary Artery Fistulas
Background
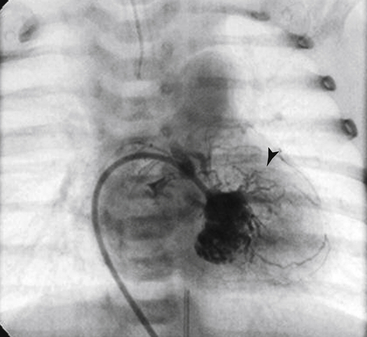
![]() Although CAFs most commonly (up to 90%) terminate in right-sided chambers or vessels (including the right atrium [RA], RV, PA, coronary sinus, superior vena cava), termination in left-sided chambers has also been reported (LA and LV).17 Coronary fistulas have been referred to as coronary–cameral fistulas; however, this phrase more appropriately describes the subset of fistulas that terminate in a cardiac chamber.18 Coronary fistulas may arise from the right coronary, left coronary, or even both vessels in the same individual.19
Although CAFs most commonly (up to 90%) terminate in right-sided chambers or vessels (including the right atrium [RA], RV, PA, coronary sinus, superior vena cava), termination in left-sided chambers has also been reported (LA and LV).17 Coronary fistulas have been referred to as coronary–cameral fistulas; however, this phrase more appropriately describes the subset of fistulas that terminate in a cardiac chamber.18 Coronary fistulas may arise from the right coronary, left coronary, or even both vessels in the same individual.19
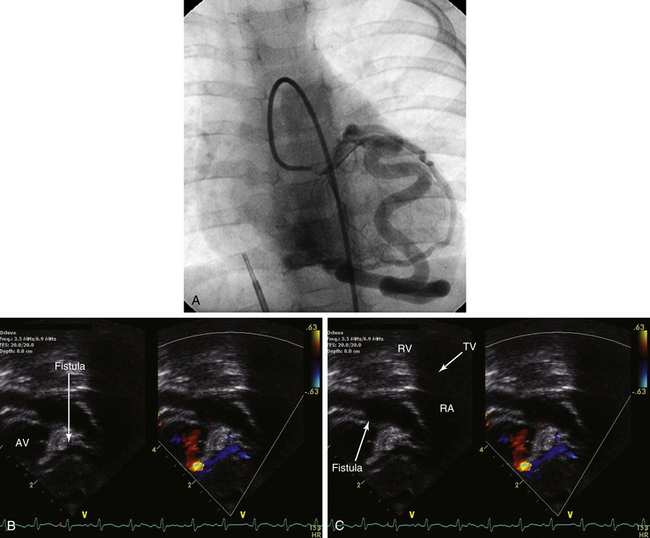
![]() (Figure 22–9 and Video 22–3).
(Figure 22–9 and Video 22–3).
Diagnosis and Clinical Presentation
Size
2008 ACC/AHA Guidelines
Small
<2 × reference vessel diameter
Class I: A small to moderate CAVF in the presence of documented myocardial ischemia, arrhythmia, otherwise unexplained ventricular systolic or diastolic dysfunction or enlargement, or endarteritis should be closed via either a transcatheter or surgical approach after delineation of its course and its potential to fully obliterate the fistula. (Level of Evidence: C)
Class IIa: Clinical follow-up with echocardiography every 3 to 5 years can be useful for patients with small, asymptomatic CAVF to exclude development of symptoms or arrhythmias or progression of size or chamber enlargement that might alter management. (Level of Evidence: C)
Class III: Patients with small, asymptomatic CAVF should not undergo closure of CAVF. (Level of Evidence: C)
Moderate
2-3 × reference vessel diameter
See above.
Large
>3 × reference vessel diameter
Class I: A large CAVF, regardless of symptomatology, should be closed via either a transcatheter or surgical route after delineation of its course and its potential to fully obliterate the fistula. (Level of Evidence: C)
![]() In contrast, small coronary fistulas may only be seen as an incidental finding on coronary angiography. Cardiac CT or MRI are generally not required for diagnosis, but they may be helpful for preprocedural planning. Nuclear myocardial perfusion may be helpful for evaluating for ischemia in the territory of the fistula resulting from coronary steal,38 especially in patients with a history of atherosclerotic coronary disease. Cardiac catheterization and angiography is the gold standard for identification of the coronary fistula
In contrast, small coronary fistulas may only be seen as an incidental finding on coronary angiography. Cardiac CT or MRI are generally not required for diagnosis, but they may be helpful for preprocedural planning. Nuclear myocardial perfusion may be helpful for evaluating for ischemia in the territory of the fistula resulting from coronary steal,38 especially in patients with a history of atherosclerotic coronary disease. Cardiac catheterization and angiography is the gold standard for identification of the coronary fistula ![]() (see Figure 22–8, A, and Video 22–4). Most adult patients do not have a quantifiable left-to-right shunt by catheterization, whereas rarely, infants with an especially large coronary fistula may have a Qp:Qs greater than 1.
(see Figure 22–8, A, and Video 22–4). Most adult patients do not have a quantifiable left-to-right shunt by catheterization, whereas rarely, infants with an especially large coronary fistula may have a Qp:Qs greater than 1.
Patient Selection
Technique
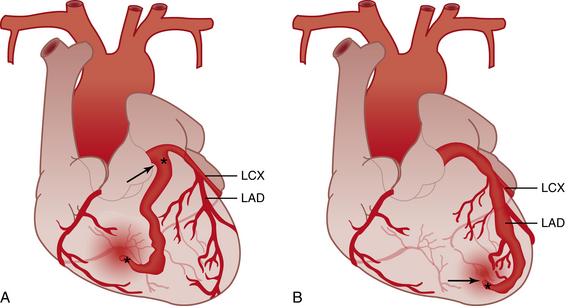
A, Proximal type coronary fistula. A vessel branches off the main coronary artery and terminates in the low-pressure chamber or vessel, becoming dilated while leaving the remainder of the main vessel normal caliber. B, Distal type coronary fistula. The distal end of the native coronary artery terminates in a low-pressure chamber or vessel, causing the entire main coronary artery to dilate. LCX, Left circumflex coronary artery; LAD, left anterior descending artery. (Modified from Gowda ST, Latson LA, Kutty S, et al: Intermediate to long-term outcome following congenital coronary artery fistulae closure with focus on thrombus formation, The Am J Cardiol 2011;107:302-308.)![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Unwanted Vascular Communications: Patent Ductus Arteriosus, Coronary Fistulas, Pulmonary Arteriovenous Malformations, and Aortopulmonary Collaterals