Unstable Coronary Syndromes
The current approach to unstable or acute coronary syndromes (ACSs) recognizes a heterogeneous clinical spectrum that ranges from unstable angina (UA) and non–ST-elevation myocardial infarction (NSTEMI) to ST-elevation myocardial infarction (STEMI). ACS is one manifestation of atherothrombotic disease. Other clinical manifestations of vascular disease include ischemic/embolic stroke, transient ischemic attack, renal insufficiency/failure, and limb ischemia/claudication. There is considerable overlap in the burden of vascular disease, so the presence of atherothrombosis in one vascular bed should raise the suspicion for disease in another vascular bed. As an example, an individual who has limb claudication is also likely to have coronary artery disease and should undergo equally aggressive atherothrombotic risk factor modification.
Various classifications have been used to describe the syndrome of UA. The Braunwald classification of UA is a widely used mechanism for providing diagnostic and prognostic information about the patient. In this system, angina is divided into acute rest (class III), subacute rest (class II), or exertional angina (class I). Acute rest angina is chest pain that occurred at rest within 48 hours of presentation, while subacute rest angina is chest pain that occurred at rest within the previous month, although more than 48 hours prior to presentation. Exertional angina is chest pain that has been present for <2 months’ duration that is described as new onset, severe, or accelerating in nature. This type of angina occurs with any exertion or less exertion than would normally bring about chest pain, with no rest angina for the previous 2 months. The Braunwald classification system also describes the clinical circumstances in which the angina is occurring. For example, secondary UA is caused by a clinical process that causes demand ischemia, such as gastrointestinal bleeding resulting in tachycardia. This process is in contrast to primary UA, in which supply ischemia results from plaque rupture with partial or total coronary occlusion. Postinfarction angina is a special clinical circumstance to consider, as these patients are at higher risk for adverse cardiac outcomes.
The classification for ACS focuses on electrocardiographic (ECG) findings in the first minutes to hours of an event. This approach ensures that the most appropriate management (i.e., early invasive therapy with appropriate coronary revascularization vs. a more conservative approach) occurs as rapidly as possible. Older ACS terminology is inefficient, as it focused on the ECG findings after the completion of the coronary event. Historical terms such as Q-wave and non–Q-wave myocardial infarction (MI) should therefore be avoided.
This chapter discusses the spectrum that encompasses UA and NSTEMI, while another chapter focuses on STEMI. Other causes of chest pain syndromes such as aortic dissection, acute pericarditis, or pulmonary embolus are not discussed here. These chapters follow the American College of Cardiology/American Heart Association (ACC/AHA) guidelines (Table 40.1).
TABLE
40.1 ACC/AHA Classification for Recommendations

From Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157, with permission from Elsevier.
EPIDEMIOLOGY AND PROGNOSIS
There are over 5 million annual visits to emergency departments in this country for the evaluation of chest pain, with approximately 1.5 million hospitalizations for UA/NSTEMI. This number is expected to increase over the next decade. Atherothrombotic disease, especially ACS, significantly shortens an individual’s life span. A survived acute MI shortens the expected life expectancy for a 60-year-old individual by approximately 9 years, whereas a cerebrovascular accident shortens an individual’s expected life span by about 12 years. Coronary disease is the single largest cause of mortality worldwide. This translates into approximately one of every five deaths in the United States being attributable to atherothrombotic disease. The shift in the epidemiology of coronary disease in this country, with improved overall mortality, is due to effective prevention and treatment of cardiovascular disease over the last several decades.
Most ACSs occur in individuals >65 years old, and nearly 50% occur in women. When women present with chest pain, the etiology is less likely to be secondary to obstructive coronary disease, and when coronary disease is present, it tends to be less severe than in men. In-hospital mortality of UA and NSTEMI patients is less than that of STEMI patients, although because the former are at risk for recurrent events, their long-term risk is equivalent or worse compared to STEMI patients.
CLINICAL PRESENTATION
UA and NSTEMI patients typically present with substernal chest discomfort, described as a pressure or a heavy sensation. This more accurately describes angina than terminology such as “pain.” Symptoms typically last <30 minutes, although they may recur frequently throughout the day or evening upon minimal exertion. Symptoms may occur at rest. Angina that occurs with minimal exertion is usually relieved promptly with rest or nitroglycerin. Anginal equivalents include neck and jaw discomfort, although the most common anginal equivalent is worsening dyspnea upon exertion. Atypical findings such as nausea, vomiting, and fatigue are easily overlooked, although these symptoms should be considered as angina in diabetics, women, and the elderly. Findings that are typically not characteristic of myocardial ischemia include sharp/pleuritic pain, pain that has been present for several hours, and brief pain that lasts only a few seconds. Up to 20% of MI s are “silent” and occur without any appreciable symptoms.
ECG findings include transient ST elevations, ST depressions (horizontal or downsloping), T-wave inversions, or nonspecific changes. The ECG may also appear normal. Deep symmetric T-wave inversions predict higher risk than small T-wave inversions. Ischemic T waves may also have a biphasic appearance. Dynamic ECG changes that are obtained during an episode of chest pain are particularly valuable, especially if the changes resolve in the absence of symptoms. It is important to repeat the ECG frequently, as a non–ST-elevation ACS may progress to a STEMI. Conversely, initial ST elevations may resolve quickly, thus changing the focus of early management.
PATHOPHYSIOLOGY FOR PRIMARY AND SECONDARY CAUSES OF ANGINA
The above discussion assumes a primary coronary etiology for an unstable coronary syndrome. The corresponding pathophysiology for a primary ACS is most commonly felt to be rupture of a vulnerable plaque, and less commonly due to plaque erosion or calcific nodules. Vulnerable plaques overlie lipid-rich cores that are surrounded by thin fibrous caps. Exposure of the underlying plaque to blood is a potent activator of platelets and thrombus formation. This subsequently results in microembolization of platelet aggregates and intermittent coronary vasoconstriction. The fibrous cap can become unstable as a result of fissures caused by proteinases secreted by neighboring macrophages. As a plaque matures, the fibrous cap becomes thicker and more stable. Conversely, plaque erosion occurs over lesions rich in smooth muscle cells and proteoglycans. These areas have minimal amounts of inflammatory substrate. The calcific nodule refers to rupture of a dense, calcified matrix through a fibrous cap. These are commonly associated with healed plaques. An important observation has been that acute MIs do not occur at sites of severe coronary narrowing. Two-thirds of events that are caused by an acutely occluded coronary vessel are in the location of a previous mild stenosis (i.e., stenosis <50%).
Secondary causes of ACS that are responsible for demand ischemia should be screened for and corrected if present before proceeding down the appropriate ACS management algorithm. Secondary causes include hypertensive crises, anemia/hypovolemia, worsened chronic obstructive pulmonary disease/hypoxia, hyperthyroidism, arteriovenous fistula in dialysis patients, and systemic infection. Aortic stenosis and hypertrophic obstructive cardiomyopathy are cardiac diseases that may cause demand myocardial ischemia. Cocaine use is a special condition to consider, as it can produce both demand ischemia (increased heart rate and blood pressure) as well as supply ischemia (coronary vasospasm and thrombus formation).
RISK STRATIFICATION
ECG
Risk stratification is a necessary component in the initial management of coronary disease patients. The ECG is a first-line test that provides not only diagnostic but also prognostic information. Data from the Global Use Of Strategies To Open Occluded Arteries In Acute Coronary Syndromes (GUSTO IIb) trial revealed a lower 6-month survival among patients with ST depressions treated conservatively compared to STEMI patients treated with fibrinolysis. The lowest-risk ACS patients (among those with any ECG changes) were those with T-wave inversions. Patients with a completely normal ECG had the lowest overall risk. A similar analysis from the relationship between insulin sensitivity and cardiovascular disease risk (RISC) Study Group found the highest risk to be among those with ST elevations and reciprocal changes (ST depressions). The lowest risk was among those with no ST changes or nonspecific ST-T changes.
Biomarkers
While the ECG is being performed and interpreted, blood work should be sent for analysis of complete blood count (CBC), chemistry, cardiac biomarkers, markers of inflammation and volume overload, and a lipid profile. Although it is not usually thought of for this purpose, information available from the CBC can be helpful in providing a crude measure of risk stratification. An elevated white blood cell (WBC) count has been shown to predict worse cardiac outcomes in ACS patients.
High-sensitivity C-reactive protein (hs-CRP) as a marker of inflammation provides prognostic information in unstable coronary syndromes. An elevated hs-CRP predicts a three- to fourfold increased risk for future cardiac events. This increased risk for MI can be attenuated by the use of aspirin. An hs-CRP level >3 mg/L is considered high risk, while a level >10 mg/L is considered an acute-phase response and should be repeated in 3 weeks. An elevated CRP predicts future cardiac events better than elevated cholesterol or presence of the metabolic syndrome.
An elevated troponin I or T also carries independent prognostic information. A meta-analysis showed that troponin-positive ACS patients have a fourfold increased risk for death compared to troponin-negative patients. Similarly, an analysis of the thrombolysis in myocardial infarction (TIMI) IIIb trial documented an eightfold increased risk of death at 42 days for patients with an elevated troponin I (>9 ng/mL) compared to troponinnegative patients. The 42-day mortality in patients with an elevated troponin was 7.5%, compared to 1% in those with a negative troponin.
An elevated brain natriuretic peptide (BNP) predicts increased risk for adverse cardiac events across the spectrum of ACS, although the predictive effect is greatest for UA and NSTEMI. BNP is a cardiac neurohormone synthesized in the ventricles and released as a larger peptide which is then cleaved into smaller portions including BNP and inactive N-terminal proBNP peptide (NT-proBNP). The release of BNP reflects the decompensated state of the ventricles, and it causes vasodilatation, natriuresis, and diuresis, leading to some improvement of the loading conditions of the failing heart. Even though BNP is the active hormone, both forms can be measured and serve as markers of congestive heart failure (CHF).
The combination of multiple biomarkers has incremental value. An hs-CRP combined with cardiac biomarkers (i.e., troponin I or T) and markers of pressure/volume overload (i.e., BNP) predict an increased risk for major cardiac events. In the OPUS-TIMI 16 trial there was a sixfold increased risk for 30-day cardiac events when all three markers were elevated. Similarly, in the TACTICS-TIMI 18 trial there was a 13-fold increased risk in 30-day cardiac events. So hs-CRP, troponin I or T, and BNP provide prognostic information in ACS patients. Other inflammatory markers such as CD-40 ligand are experimental, although they may have a role in the future in predicting the overall risk for cardiac events in ACS.
Risk Scores
The TIMI risk score incorporates data derived from the TIMI 11B trial and has been validated by three additional trials. The TIMI risk score is an easily used model that has important prognostic and therapeutic implications. It incorporates seven variables that are readily available from the history, ECG, and cardiac biomarkers (Fig. 40.1). The presence of six or seven risk factors predicts a 40% incidence of death, MI, or ischemia requiring repeat revascularization by 30 days. This is in contrast to zero or one risk factor, where the 30-day cardiac event rate is <5%. The seven variables used to calculate the TIMI risk score are age ≥65 years, ≥3 coronary disease risk factors (defined as diabetes, hypertension, hyperlipidemia, use of tobacco, and family history of premature coronary disease), a known coronary stenosis of >50%, ST deviation (transient ST elevations, ST depressions, or T-wave inversions), ≥2 anginal events in the past 24 hours, aspirin use in the last 7 days, and elevated cardiac biomarkers (i.e., elevated CK-MB or troponin).
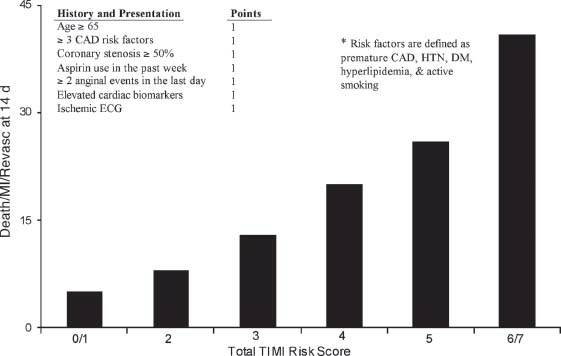
FIGURE 40.1 TIMI risk model for prediction of short-term adverse cardiac events in UA/NSTEMI patients. (Adapted from Antman EM, Cohen M, Bernink PJ, et al. The TIMI risk score for unstable angina/non-ST elevation MI: a method for prognostication and therapeutic decision making. JAMA. 2000;284:835–842.)
Another risk score which has been utilized extensively is the global registry of acute coronary events (GRACE) score. This score is the composite of nine variables, which, when added together, can be plotted on a nomogram to determine mortality risk from discharge to 6 months. The variables included in the score include age, history of CHF, history of MI, heart rate and blood pressure on presentation, presence of ST-segment depression on initial ECG, serum creatinine and elevated cardiac biomarkers during hospitalization, and no percutaneous coronary intervention (PCI) performed during hospitalization.
With either scoring system in patients presenting with UA/NSTEMI, there is progressively greater benefit of more aggressive therapies as the risk score rises.
MANAGEMENT
Initial Approach
The initial assessment of UA/NSTEMI coronary syndromes includes establishing intravenous access and starting supplemental oxygen in patients who are hypoxic or who show signs of respiratory distress. Simultaneously, an ECG must be interpreted, a targeted history and physical exam taken, and cardiac biomarkers measured. Preferred cardiac biomarkers include troponin I or T and CK-MB. Total CK (without MB) should not be used to evaluate an ACS (class III recommendation).
The primary management focus during an ACS, while antithrombotic and anti-ischemic medicines are administered (Table 40.2), is to determine a patient’s suitability for early invasive therapy versus conservative therapy. While fibrinolytic therapy plays an invaluable role in STEMI patients, it should not be used for the management of UA/NSTEMI unstable coronary syndromes (class III recommendation).
TABLE
40.2 Class I Anti-ischemic Recommendations
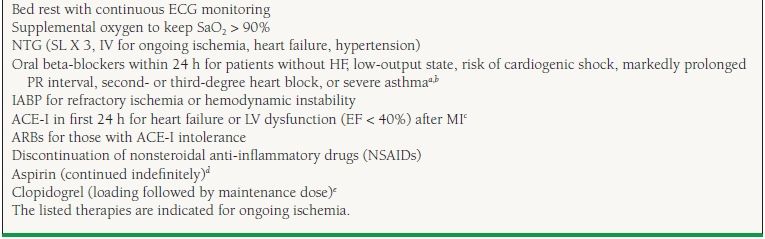
aIntravenous beta-blockers class IIa.
bNondihydropyridine calcium channel blockers may be used when beta-blockers are not successful, or there is a contraindication to their use.
cACE-I are continued when ischemia is controlled, especially for LV dysfunction or diabetes.
dClopidogrel can be substituted in patients who cannot take aspirin due to hypersensitivity or major gastrointestinal intolerance.
ePrasugrel can be substituted if planning for PCI with risk of bleeding is low and CABG considered unlikely—class IIb recommendation.
From Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157, with permission from Elsevier.
Invasive Therapy
Early trials failed to show a benefit from an invasive approach in UA/NSTEMI patients. A meta-analysis performed in the current PCI era analyzed all available studies that randomized patients to early invasive therapy versus conservative management. In those studies, patients who were treated conservatively could have an angiogram performed if they had recurrent chest pain, ischemic ECG changes, a large reversible defect with noninvasive stress testing, or elevated cardiac biomarkers. Only contemporary trials that used glycoprotein (GP) IIb/IIIa inhibitors and intracoronary stents were included. Five studies, involving nearly 7,000 UA/NSTEMI patients, were analyzed. This analysis revealed a 6- to 12-month survival advantage from early invasive therapy compared to conservative management (RR = 0.80, 95% CI 0.63 to 1.03). In contrast, studies that enrolled patients before the routine use of stents and GP IIb/IIIa inhibitors revealed a harmful association from early invasive therapy (RR 1.31, 95% CI 0.98 to 1.75).
The most contemporary large-scale data on this topic derive from the Invasive versus Conservative Treatment in Unstable coronary Syndromes (ICTUS) trial, which randomized 1,200 UA/NSTEMI patients to routine invasive or selective invasive management. Patients in the selective invasive arm were treated medically and only in cases of refractory angina or a positive exercise stress test underwent coronary angiography with or without revascularization. Results at the end of 1 year and after 3 years follow-up showed there was no significant difference in the composite ischemic end point. The investigators postulate that due to the high rate of revascularization in the selective invasive therapy arm (47%), use of aggressive medical therapy in both arms (including routine use of clopidogrel in the conservative arm) and low event rate, there was little incremental benefit to be observed with an early invasive strategy. Given the results of ICTUS, the ACC/AHA guidelines recognize that an initially conservative (selective invasive) strategy may be considered as a treatment option in stabilized UA/NSTEMI patients.
Earlier trials comparing early invasive versus conservative management include third randomized intervention treatment of angina (RITA-3) and FRagmin and fast revascularization during InStability in Coronary artery disease (FRISC II). In the RITA-3 trial, 1,810 UA/NSTEMI patients were randomized to interventional versus conservative treatment. Like ICTUS, at 1 year, death and MI rates were similar, but at 5 years, a significant reduction in death or MI emerged in the early invasive treatment arm. Benefits were seen mainly in high-risk patients. Similarly, an invasive strategy was favored at 5 years in the FRISC II trial for the primary end point of death or nonfatal MI (HR 0.81, p = 0.009). Here, the benefit was confined to males, nonsmokers, and patients with two or more cardiac risk factors.
A meta-analysis of seven randomized trials of management strategies in UA/NSTEMI, including ICTUS, supports the long-term benefit of an early invasive strategy. Among 8,375 patients, the incidence of all-cause mortality at 2 years was 4.9% in the early invasive group compared with 6.5% in the conservative groups (RR 0.75, 95% CI 0.63 to 0.90, p = 0.001), while also showing a significant reduction in nonfatal MI and hospitalization
Data from the ABOARD, TIMACS, and ISAR-COOL studies helped to determine the optimal timing of the invasive strategy. These three trials, taken together with earlier studies, do provide support for a strategy of early angiography and intervention to reduce ischemic complications in patients who have been selected for an initial invasive strategy, particularly among those at high risk (defined by GRACE score >140), whereas a more delayed approach is reasonable in low- to intermediate-risk patients. The “early” time period in this context is considered to be within the first 24 hours after hospital presentation, although there is no evidence that incremental benefit is derived by angiography and intervention performed within the first few hours of hospital admission. The advantage of early intervention was achieved in the context of intensive background antithrombotic therapy.
Therefore, current ACC/AHA guidelines recommend (class I) an early invasive approach to patients with angina in the presence of heart failure symptoms (pulmonary edema, an S3 gallop, or new mitral regurgitation), known left ventricular dysfunction, hemodynamic instability, positive noninvasive stress test (large area of ischemia), sustained ventricular tachycardia, or prior revascularization (prior coronary artery bypass grafting [CABG], or PCI within the last 6 months). The updated guidelines additionally recommend that individuals with rest angina despite intensive anti-ischemic therapy or with new ST depressions or elevated cardiac biomarkers be directed to early invasive therapy. Routine invasive therapy is discouraged in low-risk patients and those with extensive comorbidities (class III recommendation).
Intermediate-risk patients can initially be treated by either an early invasive or a conservative approach with careful monitoring for the development of high-risk features. High-risk features include refractory pain, angina with dynamic ECG changes, or elevated cardiac biomarkers. Such a change in clinical status should advance therapy to a more invasive approach along with adjunctive GP IIb/IIIa inhibitor use.
Low-risk patients can often be treated as outpatients or screened for MI with serial cardiac enzymes in a chest pain unit with a goal of early discharge. Invasive therapy is discouraged in these patients. Risk-factor modification is emphasized to all patients regardless of their risk at presentation.
Once the decision is made to perform coronary angiography, the patient’s suitability for coronary revascularization is determined. Two options for revascularization are PCI (i.e., percutaneous transluminal coronary angioplasty [PTCA] and intracoronary stents) or CABG. The choice of which revascularization to perform is beyond the scope of this chapter, although several general guidelines exist. Severe left main trunk disease is usually an indication for CABG, although left main PCI can be performed in select cases (i.e., the patient is not a candidate for open heart surgery). Severe three-vessel disease or severe two-vessel disease involving the left anterior descending artery, along with left ventricular dysfunction or diabetes, also favor CABG (Fig. 40.2).
FIGURE 40.2 Revascularization strategy in UA/NSTEMI. *There is conflicting information about these patients. Most consider CABG to be preferable to PCI. CABG, coronary artery bypass graft; LAD, left anterior descending coronary artery; PCI, percutaneous coronary intervention; UA/NSTEMI, unstable angina/non–ST-elevation myocardial infarction. (From Anderson JL, Adams CD, Antman EM, et al. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-Elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol. 2007;50(7):e1–e157, with permission from Elsevier.)



