Unfractionated Heparin
Heidar Arjomand
Marc Cohen
Plaque rupture and arterial injury at the site of percutaneous coronary intervention (PCI) expose the thrombogenic subintimal layers to platelets, activates the coagulation system, and serves as a potent stimulus for thrombin formation; hence, anticoagulant and antiplatelet agents are routinely administered during PCI to reduce thrombotic complications. Since the advent of coronary angioplasty (1), intravenous unfractionated heparin (UFH) has remained the primary antithrombotic therapy to prevent periprocedural ischemic complications (1,2). In the last few years, the widespread use of stents, thienopyridines, and glycoprotein (GP) IIb/IIIa antagonists have decreased the ischemic complications of PCI substantially. There is a relative paucity of randomized trial data on the role of UFH in PCI (3). Current recommendations on the use of intravenous UFH in PCI are empiric, and information concerning the optimal levels and duration of anticoagulation come from retrospective analyses (2). This chapter reviews the data on the role of UFH during coronary interventions.
UNFRACTIONATED HEPARIN
Unfractionated heparin is a heterogeneous mixture of polysaccharide chains, which binds to antithrombin to potentiate the inhibition of thrombin and factor Xa (4). Heparin was first used in 1937, to prevent venous thrombosis in surgical patients. Since then, heparin has acquired a major role in cardiovascular therapy; it is the routine anticoagulant agent used in percutaneous transluminal coronary angioplasty (PTCA) and coronary artery bypass graft (CABG) surgery and has confirmed value in the management of patients with acute coronary syndromes and as adjunctive therapy in patients receiving thrombolysis after myocardial infarction (MI). Despite its various limitations, intravenous UFH still is used widely during PCI.
In the cardiac catheterization laboratory, heparin effect is measured by the activated clotting time (ACT), which is the time for whole blood to form a clot in response to diatomaceous earth (Hemochron, International Technidyne Corporation, Edison, New Jersey) or kaolin (HemoTec, Medtronic-HemoTec, Johnston, Rhode Island) (5). The two systems yield different values when exposed to the same blood sample. Avendano and Ferguson found that the Hemochron ACT was consistently higher (mean 100 ± 86 seconds) than the HemoTec ACT in paired blood samples from patients undergoing PCI (6). ACT levels vary substantially after a fixed-dose bolus of heparin due to variation in body size (7), anginal syndrome (8), and conditions that predispose to heparin resistance (4).
During PCI, intravenous UFH has been used both without and with GP IIb/IIIa inhibitors (9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26).
Unfractionated Heparin without Glycoprotein IIb/IIIa Inhibitors
Intravenous UFH is administered during PCI to reduce the risk of abrupt closure and decrease ischemic complications. The optimal level of anticoagulation remains a debatable issue. Initial guidelines to achieve activated clotting times of 300 to 350 seconds were derived from observations during cardiac surgery. An ACT of 300 was the lower limit recommended for cardiac surgery to prevent thrombi in the extracorporeal circuit during cardiopulmonary bypass (27), and an ACT of less than 400 was associated with the formation of microscopic fibrin monomers in rhesus monkeys during cardiopulmonary bypass (28).
Data from retrospective studies indicated an inverse relationship between the level of anticoagulation and incidence of abrupt closure and ischemic complications (9,10). Minimum ACT values correlated with efficacy of anticoagulation, whereas maximum ACT values correlated with bleeding complications. Ferguson et al. found that complications (death and emergent or urgent bypass surgery) occurred in all patients with an ACT of <250 seconds (HemoTec), whereas only 0.3% of patients with an ACT >300 seconds suffered complications (9). Using the Hemochron device, Narins et al. found that patients experiencing abrupt closure after balloon angioplasty (BA) had significantly lower median ACT values (350 seconds versus 380 seconds) and lower minimal ACT values (345 seconds versus 370 seconds) (10). On the other hand, some authors have described low event rates in patients receiving much lower doses (2,500 to 5,000 U) of heparin (11, 12, 13). A definite relationship between the level of anticoagulation and ischemic complications was not observed in these series of low-risk patients.
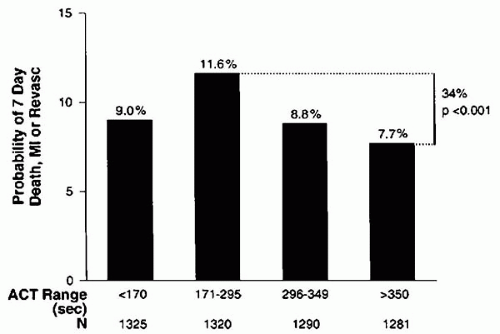
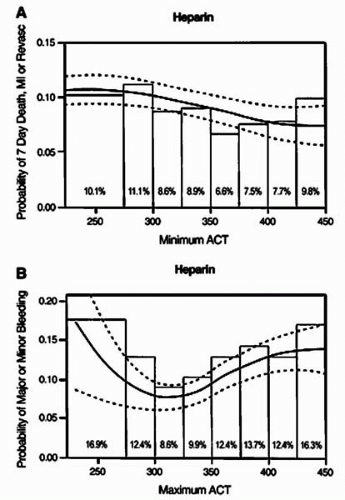
The American College of Cardiology/American Heart Association (ACC/AHA) guidelines for PCI recommends sufficient heparin to achieve and maintain an ACT of 250 to 300 seconds with the HemoTec device and 300 to 350 seconds with the Hemochron device in patients undergoing PCI without concomitant GP IIb/IIIa inhibitors (14). It should be recognized, however, that some patients will require larger doses of heparin than others to achieve the same ACT, and this may carry an adverse prognosis. In a pooled analysis, Chew et al. reviewed the data from six randomized, controlled trials of novel adjunctive antithrombotic regimens for PCI, in which UFH constituted the control arm (15). In this analysis, they compared the event rates (death, MI, or any revascularization and major or minor bleeding) at 7 days between patients divided into groups with ACTs of 25 seconds apart. An ACT in the range of 350 to 375 seconds provided the lowest composite ischemic event rate of 7.7%, compared to 11.6% when the ACT was between 171 to 295 seconds (Figs. 46.1 and 46.2). Beyond an ACT of >475 seconds, however, an increase in event rate occurred (9.3%) (15). Moreover, a direct correlation was observed between ACT levels and the rates of minor and major bleeding (Fig. 46.2B).
Several randomized clinical trials demonstrated that prolonged therapy with UFH after successful PCI has no effect on acute ischemic endpoints (16, 17, 18). In a study by Ellis et al., heparin infusion for 18 to 24 hours after successful intervention was associated with a doubling in the rate of major bleeding (3.8% versus 8.2%) with no significant change in the rate of acute occlusion (2.4% versus 1.8%) (16). Similarly, in a randomized, multicenter trial of 414 patients, the Heparin after Percutaneous Intervention (HAPI) trial, heparin therapy after successful PCI was associated with a significant increase in the rate of bleeding even if the vascular sheaths were removed early (18). Therefore, it is well established that prolonged anticoagulation and delayed sheath removal results in an increased risk
for bleeding and vascular complications (16, 17, 18). However, the role of prolonged therapy with UFH in unsuccessful PCI is unknown. The minimum safe ACT level for stent implantation in the absence of concomitant therapy with GP IIb/IIIa inhibitors is not yet determined. In the aforementioned, pooled data analysis, Chew et al. studied a subgroup of patients receiving stents. Their analysis revealed the lowest rates of ischemic events with ACTs of 350 to 375 seconds (15). At the other extreme, Colombo et al. reported a low rate of combined acute and subacute stent thrombosis of 0.9% in patients undergoing Palmaz-Shcatz stent implantation, who had optimal stent expansion by intravascular ultrasound (IVUS) and were treated with antiplatelet therapy alone (19). It should be recognized, however, that patients in the study by Chew et al. constituted a high-risk group (15).
for bleeding and vascular complications (16, 17, 18). However, the role of prolonged therapy with UFH in unsuccessful PCI is unknown. The minimum safe ACT level for stent implantation in the absence of concomitant therapy with GP IIb/IIIa inhibitors is not yet determined. In the aforementioned, pooled data analysis, Chew et al. studied a subgroup of patients receiving stents. Their analysis revealed the lowest rates of ischemic events with ACTs of 350 to 375 seconds (15). At the other extreme, Colombo et al. reported a low rate of combined acute and subacute stent thrombosis of 0.9% in patients undergoing Palmaz-Shcatz stent implantation, who had optimal stent expansion by intravascular ultrasound (IVUS) and were treated with antiplatelet therapy alone (19). It should be recognized, however, that patients in the study by Chew et al. constituted a high-risk group (15).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree