Fig. 4.1
Examples of ultrasound image (a) and of echo-tracking radio-frequency image (b) of the carotid vessels
The normal IMT values are influenced by age and sex. IMT normal values may be defined in terms of statistical distribution within a healthy population [9].
More recently, C-IMT reference values by echo-tracking were obtained in a cohort of 24,871 individuals from 24 research centers worldwide [8] and allowed estimation of C-IMT age- and sex-specific percentiles in a healthy population of the 4,234 individuals without CV disease, CV risk factors, and blood pressure-, lipid-, and/or glucose-lowering medication. These reference values will favor the use of C-IMT assessment in clinical practice, possibly for a better risk classification particularly concerning C-IMT modifications over time.
The increase in IMT may be better defined in terms of increased risk, and available data indicate that IMT > 0.9 mm, by the use of caliper measurements of B-mode images, represents a risk of myocardial infarction and/or cerebrovascular disease [10–16]. No data are presently available on IMT values obtained by the echo-tracking technique as related to the risk of CV events.
Ultrasound may also identify the presence of plaques, defined by the Manheim consensus [2] as a focal structure encroaching into the arterial lumen of at least 0.5 mm or 50 % of the surrounding IMT value, or demonstrate a thickness >1.5 mm as measured from the media-adventitia interface to the intima-lumen interface.
Ultrasonic plaque morphology may add useful information on plaque stability and may correlate with symptoms. In addition to the visual judgment of plaque echolucency and homogeneity, the use of noninvasive methods that may quantify tissue composition of vascular wall (such as videodensitometry or the analysis of integrated backscatter signal) has been proposed for the assessment of cellular composition of atherosclerotic plaque, particularly of earlier lesions [17, 18].
In addition, plaque volume assessment by three-dimensional reconstruction of ultrasound or nuclear magnetic resonance images has been proposed to better evaluate atherosclerotic lesions changes and stratify patients’ risk [19, 20] (Fig. 4.2).
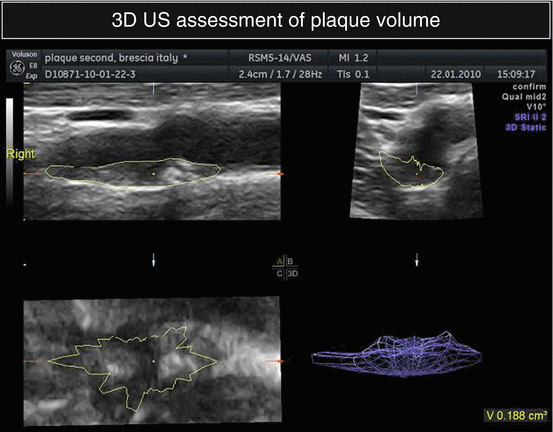

Fig. 4.2
3D reconstruction of a carotid plaque
4.2 Prevalence
Population studies, such as the Rotterdam [10] and the Cardiovascular Health Study [16] or the Vobarno study [21], have clearly demonstrated that systolic blood pressure is a major determinant of the increase of intima-media thickness in the carotid arteries, particularly in hypertensive patients.
Data collected in the VHAS (Verapamil in Hypertension and Atherosclerosis Study) [22] and the ELSA studies [23] have shown a high prevalence of carotid wall structural changes in hypertensive patients; in the VHAS study, 40 % of the patients had a plaque (i.e., an intima-media thickness >1.5 mm) in at least 1 site along the carotid arteries, and only 33 % of patients had normal carotid arteries walls. In the ELSA study, 82 % of 2,259 essential hypertensives had a plaque (i.e., an intima-media ≥ 1.3 mm). Moreover, in the RIS study (Risk Intervention Study), patients with severe essential hypertension and high cardiovascular risk had a significantly higher prevalence of atherosclerotic lesions in respect to control subjects [24]. In patients with resistant hypertension, the presence of a carotid plaque has been identified in up to 97 % of subjects [25].
4.3 Change with Treatment (Criteria for Significant Change, Incidence During Treatment)
Therapeutic double-blind trials have shown that antihypertensive drugs may have a more or less marked effect on carotid IMT progression. A large meta-regression analysis [26], including 22 randomized controlled trials, has evaluated the effects of an antihypertensive drug versus placebo or another antihypertensive agent of a different class on carotid intima-media thickness. The results have shown that, compared with no treatment, diuretics/±beta-blockers, or ACE inhibitors, CCBs attenuate the rate of progression of carotid intima-media thickening. In the prevention of carotid intima-media thickening, calcium antagonists are more effective than ACE inhibitors, which in turn are more effective than placebo or no treatment, but not more active than diuretics/±beta-blockers. The odds ratio for all fatal and nonfatal cardiovascular events in trials comparing active treatment with placebo reached statistical significance (P = 0.007).
Few studies, including a relatively small number of patients, have shown a lower thickness of intima-media during treatment with angiotensin II antagonists in respect with patients treated with beta-blockers [27].
Several randomized, comparative studies performed in the late 1990s have shown an effect of statin treatment on IMT progression. A first meta-analysis published in 2004 [28], evaluating 10 trials with 3,443 individuals (age range from 30 to 70 years) and follow-up for 1–4 years, has shown that conventional statin treatment reduces IMT progression as compared to placebo (8 studies) and that aggressive cholesterol reduction with high-dose statin may be more effective than conventional dosages.
A more recent meta-analysis [29] has examined 21 randomized controlled trials involving 6,317 individuals. The pooled weighted mean difference between statin therapy and placebo or usual care on CCA-IMT was −0.029 mm (95 % CI: −0.045, −0.013), and subgroup analyses showed a greater decrease in mean CCA-IMT in the setting of secondary prevention versus primary prevention, in younger patients versus older patients, and in studies with a greater proportion of male patients .
In other recent studies (ENHANCE, RADIANCE1, and RADIANCE2), no significant differences in IMT between treatment groups were observed in patients with familial hypercholesterolemia, even while significant decreases in LDL levels and increased HDL were observed, perhaps because patients had been previously long-term and optimally treated with statins and no difference in IMT occurred.
The results of the PHYLLIS study have reported that in hypertensive and hypercholesterolemic patients, the administration of pravastatin prevents the progression of carotid intima-media thickness seen in patients treated with hydrochlorothiazide, but the combination of pravastatin and the ACE-inhibitor fosinopril had no additive effect [30].
The greater reduction of plaque volume with the angiotensin II blocker in respect to the beta-blocker was demonstrated by a study (Multicenter Olmesartan Atherosclerosis Regression Evaluation (MORE)) assessing the effect of long-term treatment with an AT1 receptor antagonist (olmesartan) and with a beta-blocker (atenolol) on carotid atherosclerosis, with the use of the noninvasive 3D plaque measurement [31]. A significant change in 3D plaque volume was also observed during short-term treatment with a high-dose statin in a small group of 20 patients [32].
No significant changes in plaque composition were observed after 4 years of treatment with either lacidipine or atenolol in patients participating into the ELSA study, suggesting that treatment with a calcium antagonist may slow IMT progression, without influencing the characteristics of plaque tissue [33].
4.4 Prognostic Value of Baseline and of Changes of IMT and Plaque
Traditional risk factors, including male sex, ageing, being overweight, elevated blood pressure, diabetes, smoking, are all positively associated with carotid IMT in observational and epidemiological studies. Hypertension, and particularly, high systolic BP values, seems to have the greatest effect on IMT [34].
Some new risk factors, including various lipoproteins, plasma viscosity, and hyperhomocysteinemia, have demonstrated an association with increased IMT. Patients with metabolic syndrome have higher IMT than patients with individual metabolic risk factors. Carotid IMT has also been found to be associated with preclinical cardiovascular alterations in the heart, in the brain, in the kidney, and in the lower limb arteries.
Several studies have demonstrated and confirmed the important prognostic significance of intima-media thickness, as measured by ultrasound. In their prospective study, Salonen et al. [11] have observed, in 1,288 Finnish male subjects, that the risk for coronary events was exponentially related to the increase of intima-media thickness in the common carotid and in the carotid bifurcation. In a larger sample of middle-age subjects (13,780) enrolled into the ARIC (Atherosclerotic Risk in Communities) study [12], intima-media thickness, measured by ultrasound, was associated to an increased prevalence of cardiovascular and cerebrovascular diseases. In the Rotterdam study [13], the intima-media thickness was shown to predict the risk of myocardial infarction and cerebrovascular events during a mean follow-up period of 2.7 years. The results of the CHS [16] have prospectively evaluated 4,400 subjects aged more than 65 years for a follow-up period of 6 years; the annual incidence of myocardial infarction or stroke increased in the highest quintiles of intima-media thickness measured in the common and the internal carotid arteries even in this large group of elderly subjects.
A recent meta-analysis of data collected in eight studies in general populations, including 37,197 subjects who were followed up for a mean of 5.5 years, has demonstrated that for an absolute carotid IMT difference of 0.1 mm, the future risk of myocardial infarction increases by 10–15 % and the stroke risk increases by 13–18 % [14].
A second meta-analysis that evaluated CCA-IMT alone and excluded CCA or bulb IMT or plaques in 45,828 patients from 14 population-based studies showed that the addition of C-IMT does not add clinically meaningful information to the standard prediction modalities [15]. The net reclassification index with the addition of CCA-IMT was only 0.8 % for the overall cohort and 3.6 % for those at intermediate risk.
A high heterogeneity in the assessment of C-IMT in the different studies (number of carotid segments, type of measurements, number of imaging angles, inclusion of plaques in IM thickness, the use of adjusted or unadjusted models, and the different arbitrary cutoff points for C-IMT and for plaque) may explain the different results related to risk prediction. CCA-IMT measurement at sites not containing plaque and IMT measurement in the carotid bulb and ICA, inclusive of plaque (if present), represent two separate phenotypes, with different association to the risk of cardiac and cerebrovascular events.
In fact, the assessment of carotid plaque appears to be a more powerful predictor of CV events than C-IMT alone, as suggested by a meta-analysis of 11 population-based studies including 54,336 patients [35]. The plaque number or the quantitative measurement of plaque thickness, area, or 3D volume may parallel the sensitivity in CV risk-predictive value [36] (Fig. 4.3).
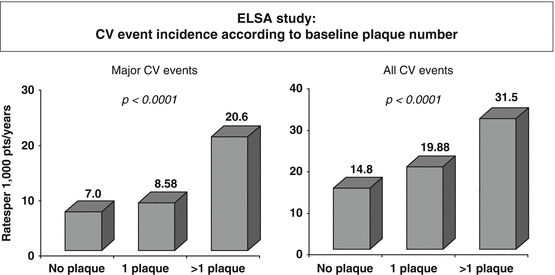

Fig. 4.3
Association of plaque number with cardiovascular events in the European Lacidipine Study on Atherosclerosis (ELSA) (Data from [39])
The additional effect of C-IMT on CV risk stratification has been confirmed in some, but not all studies. About 30 % of hypertensive subjects may be mistakenly classified as at low or moderate added risk without ultrasound for carotid arteries thickening or plaque, whereas vascular damage places them in the high added risk group [21, 34, 37].
It has been therefore proposed that IMT may be proposed as a surrogate endpoint for cardiovascular events [38]. However, available studies have not been conducted to demonstrate whether a decrease of IMT progression may be associated with a reduction of cardiovascular events and an improvement in prognosis; the retrospective analysis of some studies has given conflicting results [15, 39, 40]. It has to be acknowledged that the estimation of C-IMT in the vast majority of the studies was obtained manually by calipers, without the use of an automatic approach for the measurement, suggesting that the accuracy of C-IMT and, particularly, of its changes over time might have important consequences in the clinical setting. The use of RF C-IMT measurements has been recently shown to have an additional stratification power for coronary artery disease, in addition to the Framingham risk score [41].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


