Abstract
Bypass tracts (BTs) are remnants of the atrioventricular (AV) concaused by incomplete embryological development of the AV annuli and failure of the fibrous separation between the atria and ventricles. There are several types of BTs, according to the structures they connect, including atrioventricular, atrionodal, atrio-Hisian, atriofascicular, fasciculoventricular, and nodofascicular BTs.
In the Wolff-Parkinson-White (WPW) syndrome, AV conduction occurs, partially or entirely, through an AV BT, which results in earlier activation (preexcitation) of the ventricles than if the impulse had traveled through the AVN. Concealed AV BTs refer to AV BTs that conduct only in the retrograde direction and therefore do not result in ventricular preexcitation. AV reentrant tachycardia (AVRT) is a macroreentrant tachycardia with an anatomically defined circuit that consists of two distinct pathways, the normal AV conduction system and an AV BT, linked by common proximal (atrial) and distal (ventricular) tissues. Atrial tachycardia, atrial fibrillation, and AV nodal reentrant tachycardia can all coexist with a BT, whereby the BT serves as a bystander route for venor atrial activation.
The prevalence of WPW pattern on the surface ECG is 0.1% to 0.3% in the general population. The prevalence of the WPW syndrome (i.e., the combination of ventricular preexcitation and either a documented tachyarrhythmia or symptoms of a tachyarrhythmia) is substantially lower than that of the WPW ECG pattern. Catheter ablation is considered the treatment of choice for patients with the WPW syndrome. Catheter ablation is curative in more than 95% of patients with a relatively low complication rate.
Keywords
bypass tract, atrioventricular reentrant tachycardia, preexcitation, Wolff-Parkinson-White syndrome, permanent junctional reentrant tachycardia
Outline
Types of Bypass Tracts, 599
Atrioventricular Bypass Tracts, 599
Atrionodal Bypass Tracts, 599
Atrio-Hisian Bypass Tracts, 599
Atypical Bypass Tracts, 600
Types of Preexcitation Syndromes, 600
Wolff-Parkinson-White Syndrome, 600
Concealed Bypass Tracts, 600
Lown-Ganong-Levine Syndrome, 600
Mahaim Variant of Preexcitation, 600
Pathophysiology, 600
Wolff-Parkinson-White Syndrome, 600
Atrioventricular Bypass Tracts, 601
Atrioventricular Reentry, 602
Other Arrhythmias Associated With Wolff-Parkinson-White Syndrome, 603
Epidemiology and Natural History, 606
Wolff-Parkinson-White Pattern, 606
Wolff-Parkinson-White Syndrome, 606
Sudden Death, 606
Associated Cardiac Abnormalities, 606
Familial Wolff-Parkinson-White Syndrome, 606
Concealed Bypass Tracts, 607
Clinical Presentation, 607
Initial Evaluation, 607
Methods for Evaluation of Bypass Tract Refractory Period, 607
Principles of Management, 608
Electrocardiographic Features, 612
Electrocardiography of Preexcitation, 612
Supraventricular Tachyarrhythmias Associated With Wolff-Parkinson-White Syndrome, 614
Electrocardiographic Localization of the Bypass Tract, 614
Electrophysiological Testing, 623
Baseline Observations During Sinus Rhythm, 623
Induction of Tachycardia, 633
Tachycardia Features, 637
Diagnostic Maneuvers During Tachycardia, 641
Diagnostic Maneuvers During Sinus Rhythm After Tachycardia Termination, 648
Exclusion of Other Arrhythmia Mechanisms, 649
Localization of the Bypass Tract, 649
Pacing From Multiple Atrial Sites, 649
Preexcitation Index, 649
Effects of Bundle Branch Block During Orthodromic Atrioventricular Reentrant Tachycardia, 652
Ventricular Entrainment During Orthodromic Atrioventricular Reentrant Tachycardia, 652
Earliest Ventricular Activation Site During Anterograde Bypass Tract Conduction, 655
Earliest Atrial Activation Site During Retrograde Bypass Tract Conduction, 655
Atrial Electrogram Polarity Reversal During Retrograde Bypass Tract Conduction, 656
Direct Recording of Bypass Tract Potential, 657
Local Atrioventricular (or Ventriculoatrial) Interval, 658
Ablation, 660
Target of Ablation, 660
Ablation Technique: General Considerations, 661
Endpoints of Ablation, 662
Ablation of Left Free-Wall Bypass Tracts, 663
Ablation of Right Free-Wall Bypass Tracts, 666
Ablation of Anteroseptal (Superoparaseptal) and Midseptal Bypass Tracts, 667
Ablation of Posteroseptal (Inferoparaseptal) Bypass Tracts, 670
Ablation of Epicardial Bypass Tracts, 672
Causes of Failed Bypass Tract Ablation, 673
Outcome, 674
Types of Bypass Tracts
Bypass tracts (BTs) are remnants of the atrioventricular (AV) connections caused by incomplete embryological development of the AV annuli and failure of the fibrous separation between the atria and ventricles. There are several types of BTs, according to the structures they connect, including AV, atrionodal, atrio-Hisian, atriofascicular, fasciculoventricular, and nodofascicular BTs.
Atrioventricular Bypass Tracts
AV BTs are strands of working myocardial cells connecting atrial and ventricular myocardium across the electrically insulating fibrofatty tissues of the AV junction bypassing the atrioventricular node (AVN)-His-Purkinje system (HPS). In the older literature, these BTs were called Kent bundles, although incorrectly (Kent described AVN-like tissue in the right atrial [RA] free wall that did not connect to the ventricle). Thus the use of the term bundle of Kent should be discouraged.
Atrionodal Bypass Tracts
Atrionodal BTs connect the atrium to the distal or compact AVN. They have been called James fibers and are of uncertain physiological significance.
Atrio-Hisian Bypass Tracts
Atrio-Hisian BTs connect the atrium to the His bundle (HB); these BTs are rare.
Atypical Bypass Tracts
The term “atypical BTs” is used here to describe variants of BTs that connect the atrium (atriofascicular BTs), AVN (nodofascicular and nodoventricular BTs), or HB (fasciculoventricular BTs) to distal Purkinje fibers or ventricular myocardium. In addition, this term encompasses slowly conducting short AV BTs and long AV BTs. Although many of those variants of BTs are sometimes referred to as “Mahaim fibers,” it is more appropriate to describe them based on their anatomic connections.
Types of Preexcitation Syndromes
Several patterns of preexcitation can occur, depending on the anatomy of the BT and the direction in which impulses are conducted. Conduction from the atria to the ventricles normally occurs via the AVN-HPS. Patients with preexcitation have an additional or alternative pathway, the BT, which directly connects the atria and ventricles and bypasses the AVN. The term syndrome is used when the anatomical variant is responsible for tachycardia.
Wolff-Parkinson-White Syndrome
In the Wolff-Parkinson-White (WPW) syndrome, AV conduction occurs, partially or entirely, through an AV BT, which results in earlier activation (preexcitation) of the ventricles than if the impulse had traveled through the AVN.
Concealed Bypass Tracts
Concealed AV BTs refer to AV BTs that do not manifest anterograde conduction and therefore do not result in ventricular preexcitation. Because they do not result in alteration of the QRS complex in the electrocardiogram (ECG), they cannot be detected by inspection of the surface ECG; they are called concealed . However, the concealed BT can conduct in a retrograde fashion, thereby creating a reentrant circuit with impulses traveling from the atrium to the AVN, HPS, ventricle, and then back to the atrium via the BT.
Lown-Ganong-Levine Syndrome
In the setting of Lown-Ganong-Levine (LGL) syndrome, preexcitation purportedly occurs via atrio-Hisian BTs or, alternatively, no BT is present and enhanced AVN conduction accounts for the ECG findings. The net effect is a short PR interval without delta wave or QRS prolongation. It is important to stress, however, that LGL is not a recognized syndrome with an anatomical basis, but only an ECG description, and the use of the term should be discouraged.
Mahaim Variant of Preexcitation
The so-called Mahaim variant of preexcitation does not typically result in a delta wave because these pathways, which usually terminate in the conducting system or in the ventricular myocardium close to the conducting system, conduct slowly, and the AVN-HPS has adequate time to activate most of the ventricular muscle mass.
It is worth noting that some of the older literature refers to eponymous pathways that were originally anatomically described with subsequent attempts made to correlate these structures with physiologic findings. With more recent data from intracardiac recordings, many of these correlations have been shown to be incorrect and thus the use of the eponyms adds confusion to discussions about them. For instance, the pathways described by Kent (AV nodal–like tissue at the free-wall AV valve annulus) more resemble atriofascicular fibers than they do typical AV fibers, and atriofascicular fibers in turn possess the physiology initially (incorrectly) attributed to Mahaim fibers. Table 18.1 displays some of the terminology; it is evident why use of eponymous terms is discouraged.
| Eponymous Pathway | Anatomical Description | Proposed Physiological Role/Syndrome | Actual Physiologic Role |
|---|---|---|---|
| Kent bundle | Clusters of nodal cells at nonseptal AV junction | Anomalous AV connection/WPW syndrome | None (possibly atriofascicular pathways) |
| James fiber | Atriocompact nodal connection | LGL syndrome | Enhanced AVN conduction |
| Brechenmacher fiber | Atrio-Hisian connection | LGL syndrome | Enhanced AVN conduction |
| Mahaim fiber | Connection from compact AVN to ventricle | Septal WPW pathways with decremental conduction | Probably none |
| Paladino fiber | Connection from proximal AVN to ventricle | Septal WPW pathways with decremental conduction | Probably none |
Pathophysiology
Wolff-Parkinson-White Syndrome
WPW pattern refers to the constellation of ECG abnormalities related to the presence of a manifest AV BT (i.e., ventricular preexcitation: short PR interval, delta wave, wide QRS complex) in asymptomatic patients ( Fig. 18.1 ). WPW syndrome refers to the combination of ventricular preexcitation and either a documented tachyarrhythmia or symptoms of a tachyarrhythmia.

Because the AV BT typically conducts faster than the AVN, the onset of ventricular activation is earlier than if depolarization occurred only via the AVN, resulting in a shortened PR (P-delta) interval. Furthermore, because the BT exhibits practically nondecremental conduction, the early ventricular activation (i.e., P-delta interval) remains almost constant at all atrial rates ( Fig. 18.2 ).

Preexcited intraventricular conduction in WPW propagates from the insertion point of the AV BT in the ventricular myocardium via direct muscle-to-muscle conduction. This process is inherently slower than ventricular depolarization resulting from rapid HPS conduction. Thus, although the initial excitation of the ventricles (via the BT) occurs earlier, it is followed by slower activation of the ventricular myocardium than occurs normally. The net effect is that the QRS complex consists of fusion between the early ventricular activation caused by preexcitation with the later ventricular activation resulting from impulse propagation through the AVN-HPS to the ventricles. The initial part of ventricular activation resulting in the upstroke of the QRS complex is slurred because of slow muscle-to-muscle conduction; this is termed a delta wave .
Depending on the relative contribution from ventricular activation by the normal AVN-HPS versus the manifest BT, a variable degree of preexcitation occurs. The more rapid the conduction along the BT in relation to the AVN, the greater the amount of myocardium depolarized via the BT, resulting in a more prominent or wider delta wave and increasing prolongation of the QRS complex duration.
Atrioventricular Bypass Tracts
The cardiac skeleton consists of four rings of dense connective tissue that surround the AV canals (mitral and tricuspid) and extend to the origins of the aorta and the pulmonary trunk, providing structure and support for the heart as well as electrical isolation between the atria and the ventricles. The aortic valve occupies the central position with the other valve rings attached to it. The right fibrous trigone includes the triangular formation between the aortic valve and the medial parts of the tricuspid and mitral valves, and it represents the largest thickening and strongest portion of the cardiac skeleton. Together with the membranous septum, the right fibrous trigone constitutes the central fibrous body ( see Fig. 9.1 ).
The AV junctions are the areas of the heart where the atrial musculature connects to the annuli of the mitral and tricuspid valves. The AVN-HPS, which lies in the septal component of the AV junction, is the only normal electrical connection between the atria and the ventricles. The fibrous skeleton and AV valvular annuli (annulus fibrosus) act as insulators to prevent electrical impulses from conducting to the ventricles by any other route. The main function of the AVN is modulation of atrial impulse transmission to the ventricles, thereby coordinating atrial and ventricular contractions; it receives, delays, and conveys atrial impulses to the ventricles.
AV BTs are aberrant muscle bundles that connect the atria to the ventricles outside of the normal AV conduction system. AV BTs are found most often in the parietal AV junctional areas, including the paraseptal areas. They breach the insulation provided by the fibrofatty tissues of the AV groove (sulcus tissue) and the hinge lines (fibrous annulus) of the valves. They are rarely found in the area of fibrous continuity between the aortic and mitral valves because in this area there is usually a wide gap between the atrial myocardium and ventricular myocardium to accommodate the aortic outflow tract. The remainder of the AV groove may be divided into quadrants consisting of the left free wall, right free wall, and posteroseptal and anteroseptal spaces. The distribution of BTs within these regions is not homogeneous—46% to 60% of BTs are found within the left free-wall space; 25% are within the posteroseptal space; 13% to 21% of BTs are within the right free-wall space; up to 7% are within the right superoparaseptal (formerly called anteroseptal) space; and less than 5% are located in the midseptum ( Fig. 18.3 ).

AV BTs are usually short and very thin muscular strands (typically 5 to 10 mm in length, with a maximal diameter of 0.1 to 7 mm) but can occasionally exist as broad bands of tissue. They can course through the AV groove at variable depths ranging from subepicardial to subendocardial locations. The AV BT can run in an oblique course rather than perpendicular to the transverse plane of the AV groove. As a result, the fibers can have an atrial insertion point that is transversely from less than one to several centimeters removed from the point of ventricular attachment. Some posteroseptal pathways insert into coronary sinus (CS) musculature rather than atrial myocardium and can be associated with the coronary venous system or diverticula from a CS branch vein.
Multiple AV BTs occur in 5% to 10% of patients. BTs are defined as multiple when they are separated by more than 1 to 3 cm at the AV junction. The most common combination of widely spaced multiple BTs is posteroseptal and right free-wall BTs. The incidence of multiple BTs is particularly high in patients with antidromic atrioventricular reentrant tachycardia (AVRT) (50% to 75%), patients in whom atrial fibrillation (AF) resulted in ventricular fibrillation (VF), and patients with Ebstein anomaly.
Although the majority (approximately 60%) of AV BTs conduct both anterogradely and retrogradely (i.e., bidirectionally), some AV BTs are capable of propagating impulses in only one direction. BTs that conduct only in the anterograde direction are uncommon (less than 5%), often cross the right AV groove, and frequently possess decremental conduction properties. On the other hand, BTs that conduct only in the retrograde direction occur more frequently, accounting for 17% to 37% of all BTs. When the BT is capable of anterograde conduction, ventricular preexcitation is usually evident during normal sinus rhythm (NSR), and the BT is referred to as manifest. BTs capable of retrograde-only conduction are referred to as concealed.
Because working myocardial cells make up the vast majority of AV BTs, conduction over those BTs is mediated by the rapid inward sodium current, similar to normal His-Purkinje tissue and atrial and ventricular myocardium. Therefore AV BTs have rather constant anterograde and retrograde conduction at all rates until the refractory period is reached, at which time conduction is completely blocked. Thus conduction over AV BTs usually behaves in an all-or-none fashion (i.e., nondecremental conduction), although with careful measurement, there is often a small amount of prolongation of conduction intervals (10 to 15 milliseconds) over the BT when the pacing rate is just below that at which block occurs. In contrast, the AVN, which depends on the slow inward calcium current for generation and propagation of its action potential, exhibits what has been called decremental conduction , whereby conduction time of the impulse propagating through the AVN prolongs as the atrial cycle length (CL) shortens. Thus AV conduction is more rapid through the AV BT than through the AVN, a difference that is exaggerated at faster heart rates. This difference has potentially great clinical importance. A primary function of the AVN is to limit the number of impulses conducted from the atria to the ventricles, which is particularly important during fast atrial rates (e.g., AF or atrial flutter [AFL]) when only a fraction of impulses are conducted to the ventricles, whereas the remainder are blocked in the AVN. However, in the presence of nondecrementally conducting AV BTs with short refractory periods, these arrhythmias can lead to very fast ventricular rates that can degenerate into VF.
Atrioventricular Reentry
AVRT is a macroreentrant tachycardia with an anatomically defined circuit that consists of two distinct pathways, the normal AV conduction system and an AV BT, linked by common proximal (atrial) and distal (ventricular) tissues. If sufficient differences in conduction time and refractoriness exist between the normal conduction system and the BT, a properly timed premature impulse of atrial or ventricular origin can initiate reentry. AVRTs are the most common (80%) tachycardias associated with the WPW syndrome. AVRT is divided into orthodromic and antidromic according to the direction of conduction in the AVN-HPS ( Fig. 18.4 ). Orthodromic indicates normal direction (anterograde) of conduction over AVN-HPS during AVRT.

Orthodromic Atrioventricular Reentrant Tachycardia
In orthodromic AVRT, the AVN-HPS serves as the anterograde limb of the reentrant circuit (i.e., the pathway that conducts the impulse from the atria to the ventricles), whereas an AV BT serves as the retrograde limb (see Figs. 18.4 and 18.5 ). Approximately 50% of BTs participating in orthodromic AVRT are manifest (able to conduct bidirectionally) and 50% are concealed (able to conduct retrogradely only). Therefore a WPW pattern may or may not be present on the surface ECG during NSR. When preexcitation is present, the delta wave seen during NSR is lost during orthodromic AVRT because anterograde conduction during the tachycardia occurs over the normal AV conduction system, and not via the BT (i.e., the ventricle is not preexcited). Orthodromic AVRT accounts for approximately 90% to 95% of AVRT episodes in patients with a manifest BT and 35% of all paroxysmal supraventricular tachycardias (SVTs).

Antidromic Atrioventricular Reentrant Tachycardia
Antidromic AVRT is a preexcited AVRT whereby an AV BT serves as the anterograde limb and the AVN-HPS serves as the retrograde limb of the reentrant circuit (see Figs. 18.4 and 18.5 ). Consequently, the QRS complex during antidromic AVRT is fully preexcited (i.e., the ventricles are activated totally by the BT with no contribution from the normal conduction system). The BT involved in the antidromic AVRT circuit must be capable of anterograde conduction and, therefore, preexcitation is typically observed during NSR. Other, less frequent, forms of preexcited AVRT utilize one AV BT as the anterograde conduction and a second BT for retrograde conduction or a combination of one BT plus the AVN-HPS in either direction ( eFig. 18.1 ).

Clinically, antidromic AVRT is much less frequent than orthodromic AVRT, occurring in less than 5% of patients with WPW syndrome, and can be induced in the electrophysiology (EP) laboratory in less than 10%. The low prevalence of antidromic AVRT is related to the EP properties of the AVN, because good retrograde ventriculoatrial (VA) conduction is required to sustain tachycardia. Clinical presentation, sex, age, and orthodromic AVRT induction appear similar in patients with and without inducible antidromic AVRT. Patients with antidromic AVRT induction more frequently have BTs with more rapid conduction properties than other WPW patients.
Susceptibility to antidromic AVRT appears to be facilitated by a distance of at least 4 cm between the BT and the normal AV conduction system. Consequently, most antidromic AVRTs use a lateral (right or left) BT as the anterograde route for conduction. Because posteroseptal BTs are in close proximity to the AVN, those BTs are rarely part of antidromic AVRT if the other limb is the AVN and not a second free-wall BT. Up to 50% to 75% of patients with spontaneous antidromic AVRT have multiple BTs (manifest or concealed), which may or may not be utilized as the retrograde limb during the tachycardia.
Permanent Junctional Reciprocating Tachycardia
Permanent junctional reciprocating tachycardia (PJRT) is a rare form of nearly incessant orthodromic AVRT mediated by a concealed, retrogradely conducting AV BT that has slow and decremental conduction properties. Conduction properties of this retrograde BT are slower than the anterograde conduction properties of the AVN and those of typical fast BTs found in patients with AVRT. The BT in PJRT is most often located in the posteroseptal region, although other portions of the AV groove can also harbor this unusual pathway. Because these BTs are almost always concealed and have slow conduction, all elements necessary for reentry are present at all times, and thus PJRT can be present much of the time (i.e., incessant), with only short interludes of sinus rhythm. The incessant nature of PJRT can result in tachycardia-induced cardiomyopathy.
Other Arrhythmias Associated With Wolff-Parkinson-White Syndrome
Atrial tachycardia (AT), AFL, AF, and atrioventricular nodal reentrant tachycardia (AVNRT) can all coexist with a BT. In these preexcited tachycardias, the BT serves as a bystander route for ventricular or atrial activation, and is not required for the initiation or maintenance of the arrhythmia.
Atrioventricular Nodal Reentrant Tachycardia and Atrial Tachycardia
Both AVNRT and AT can use the bystander BT to transmit impulses to the ventricle (see Fig. 18.4 ). When AVNRT occurs in the WPW syndrome, the arrhythmia can be difficult to distinguish from AVRT without EP testing.
Atrial Fibrillation
The overall incidence of AF in patients with WPW syndrome varies from 12% to 39%. AF is typically paroxysmal. Persistent AF is rare in these patients. AF is most common in patients with anterogradely conducting BTs. Patients with antidromic AVRT, multiple BTs, and BTs that have a short anterograde effective refractory period (ERP) are more prone to develop AF. In individuals with WPW, AF is often preceded by AVRT that degenerates into AF ( Fig. 18.6 ).

The frequency with which intermittent AF occurs in patients with the WPW syndrome is striking because of the low prevalence of coexisting structural heart disease or other predisposing factors for AF. This observation suggests that the AV BT itself can be related to the genesis of AF, supported by the fact that when the BT is ablated, AF may not recur.
The mechanisms by which AVRT precipitates AF are not well understood. The rapid atrial rate can cause disruption in atrial activation and reactivation, creating an EP substrate conducive to AF. The observation that most patients with BT and AF who undergo BT ablation are cured of both AVRT and AF is compatible with this hypothesis. Another possibility is that the complex geometry of networks of BTs predisposes to AF by fractionation of the activation wavefronts. Localized reentry has been recorded in some patients, using direct recordings of the activation of the BTs. Hemodynamic changes, atrial stretch caused by atrial contraction against closed AV valves during ventricular systole, can also play a role. Ablation of the BT can cure AF in more than 90% of patients; however, vulnerability to AF persists in up to 56%, and the response to atrial extrastimulation (AES) is also unaltered by ablation.
AF in younger WPW patients is usually associated with the BT and is unlikely to occur after ablation; in contrast, older patients may have recurrence of AF from causes unrelated to the BT. Nonetheless, recent studies have challenged the role of BT ablation in modulating the risk of AF particularly in adult patients, and show persistently higher rates of AF in WPW patients (hazard ratio, 4.77) compared with the general population despite catheter ablation of the BT. Trends of increased risk of AF in ablated WPW patients suggest that mechanisms other than those directly related to the presence of a BT, such as underlying atrial myopathy, may play a role in AF genesis.
Atrial Flutter
About 4% of WPW patients present with AFL. AFL is the most common (60%) regular preexcited tachycardia in patients with WPW syndrome. AFL is caused by a macroreentrant circuit within the RA and therefore exists independently of the BT, and AFL does not have the same causal association to AV BTs as AF. In some patients with WPW syndrome who develop AFL, AVRT is often the initiating event. This relationship can be mediated by contraction-excitation feedback into the atria during the AVRT.
AFL, like AF, can conduct anterogradely via a BT causing a preexcited tachycardia. Depending on the various refractory periods of the normal and pathological AV conduction pathways, AFL can potentially conduct 1:1 to the ventricles during a preexcited tachycardia, making the arrhythmia difficult to distinguish from VT ( see Fig. 12.10 ).
Ventricular Fibrillation and Sudden Cardiac Death
The mechanism of sudden cardiac death (SCD) in patients with WPW is likely the occurrence of AF or AFL with a very rapid ventricular rate, which provokes VF. Although the frequency with which AF with rapid AV conduction via a BT degenerates into VF is unknown, the incidence of SCD in patients with WPW syndrome is rather low, ranging from 0% to 0.39% annually in several large case series. The trigger for AF in this population of patients is generally an episode of AVRT. In fact, most patients who have been resuscitated from VF secondary to preexcitation have a previous history of AVRT, AF, or both. Nonetheless, SCD can be the first manifestation of WPW syndrome.
Several factors can help identify the patient with WPW who is at increased risk for VF, including symptomatic AVRT, septal location of the BT, presence of multiple BTs, and male gender. In addition, the risk of SCD associated with WPW appears highest in the first two decades of life. Nonetheless, it is clear that the most important factor for the occurrence of VF in these patients is the ability of the BT to conduct rapidly to the ventricles. This is best measured by determining the shortest and average preexcited R-R intervals during AF or, alternatively, by measuring the anterograde ERP of the BT. If the BT has a very short anterograde ERP (less than 250 milliseconds), a rapid ventricular response can occur with degeneration of the rhythm to VF. A short preexcited R-R interval during AF (≤220 milliseconds) appears to be a sensitive clinical marker for identifying children at risk for SCD, although its positive predictive value in adults is only 19% to 38%.
Drug therapy can be an additional determinant of the risk of VF in patients with preexcitation. Several pharmacological agents can potentially enhance BT conduction and increase the ventricular rate during AF and, hence, increase the risk of VF (see later).
Ventricular Tachycardia
Coexisting VT is uncommon in patients with WPW syndrome because structural heart disease is very infrequent in this young patient population. Naturally, older patients are subject to coronary artery and other diseases that can cause VT.
Epidemiology and Natural History
Wolff-Parkinson-White Pattern
The prevalence of WPW pattern on the surface ECG is 0.1% to 0.3% in the general population. The prevalence is increased to 0.55% among first-degree relatives of affected patients, suggesting a familial component. The annual incidence of newly diagnosed cases of preexcitation in the general population was substantially lower (0.004%) in a diverse population of residents from Olmsted County, Minnesota, 50% of whom were asymptomatic. The incidence in men is twice that in women, and is highest in the first year of life, with a secondary peak in young adulthood.
The WPW pattern on the surface ECG can be intermittent and can even permanently disappear with loss of anterograde but preserved retrograde conduction. Loss of preexcitation has been observed in up to 31% of adults and in 0% to 33% of children and adolescents over a 5-year time period. Intermittent and persistent loss of preexcitation may indicate that the BT has a relatively longer baseline ERP, which makes it more susceptible to age-related degenerative changes and variations in autonomic tone.
Wolff-Parkinson-White Syndrome
The prevalence of the WPW syndrome is substantially lower than that of the WPW ECG pattern. At present, it is estimated that approximately 65% of adolescents and 40% of adults over 30 years of age with a WPW pattern on a resting ECG are asymptomatic.
The occurrence of arrhythmias is related to the age at the time preexcitation is discovered and can vary with location and EP properties of the BT. AVRT manifests early in life, with an average of more than 10 years separating the time of clinical presentation of AVRT versus that of AVNRT. Up to 70% of children (8 to 12 years of age) who are asymptomatic at the time of diagnosis of WPW ECG pattern remain asymptomatic over median follow-up of 57 months. About 30% eventually develop an arrhythmic event, which can be potentially life-threatening in approximately 10% of patients. In contrast, only a minority (10%) of adults who are asymptomatic at the time of diagnosis of ventricular preexcitation develop cardiac arrhythmias over median follow-up of 67 months, which can be potentially life-threatening in approximately 5% of patients. The vast majority of patients in whom preexcitation is first uncovered after the age of 40 remain asymptomatic. In a large meta-analysis, the annual risk of developing SVT was 0.25% of WPW patients.
A male predominance among WPW patients has been observed. Furthermore, men tend to have a higher incidence of antidromic AVRT, more prevalent left-sided BTs, and shorter anterograde BT ERP. Also, men usually present with AVRT at an older age than women. In contrast, women were found to have a higher prevalence of multiple BTs, orthodromic AVRT, and right-sided BTs. In addition, Asians appear to have right free-wall BTs substantially more frequently than other races.
Sudden Death
The incidence of SCD among patients with asymptomatic preexcitation is difficult to ascertain. A large meta-analysis (including 1869 patients with asymptomatic ventricular preexcitation from 20 studies with 11,722 patient-years of follow-up) found a total of 10 cases of SCD, with SCD rates between 0 and 4.5 events per 1000 person-years of follow-up, and an overall risk of SCD in adults and children of 2.5 per 1000 person-years (or 3% to 4% over a lifetime). Italian studies reported all but one SCD event.
In general, children seem to have a numerically higher event rate than adults. The majority of victims were between the ages of 10 and 40 years. Patients with WPW who are most susceptible to SCD are symptomatic; however, SCD can be the first event in patients with asymptomatic preexcitation. Several characteristics have been reported among patients who experienced potentially life-threatening events, including younger age (less than 30 years), male gender, history of AF, prior syncope, associated congenital or other heart disease, and familial WPW. Low-risk WPW patients also showed a characteristic EP profile (older age, lower tachyarrhythmia inducibility, longer anterograde refractory ERP of BTs, and low likelihood of baseline retrograde BT conduction or multiple BTs).
Associated Cardiac Abnormalities
Most patients with AV BTs do not have coexisting structural cardiac abnormalities, except for those that are age related. The association of ventricular preexcitation with structural congenital heart defects is well recognized. Up to 20% of children with WPW also have congenital heart disease. Associated congenital abnormalities, when present, are more likely to be right sided than left sided. Ebstein anomaly is the congenital lesion most strongly associated with the WPW syndrome. As many as 10% of such patients have one or more BTs; most of these are located in the right free-wall or the right posteroseptal space. Ventricular preexcitation has also been described in patients with transposition of great arteries, pulmonary atresia, patent ductus arteriosus, tetralogy of Fallot, total anomalous pulmonary venous return, and ventricular septal defects.
Familial Wolff-Parkinson-White Syndrome
Typically, WPW syndrome occurs sporadically; however, in a minority of cases it is inherited. A familial form of WPW has infrequently been reported and is usually inherited as an autosomal dominant trait. Among patients with the WPW syndrome, 3.4% have first-degree relatives with a preexcitation syndrome. The genetic cause of a rare form of familial WPW syndrome has been described. The clinical phenotype is characterized by the presence of preexcitation on the ECG, frequent SVTs (including AF), progressive conduction system disease, and left ventricular (LV) hypertrophy (distinct from sarcomeric hypertrophic cardiomyopathy). Patients typically present in late adolescence or the third decade with syncope or palpitations. Premature SCD occurred in 10% of patients. Paradoxically, by the fourth decade of life, progression to advanced SND or AV block (with the loss of preexcitation) requiring pacemaker implantation was common. Approximately 80% of the patients older than 50 years had chronic AF. Causative mutations in the PRKAG2 gene were identified in these families. The PRKAG2 gene encodes the gamma-2 regulatory subunit of the adenosine monophosphate (AMP)–activated protein kinase, which is a key regulator of metabolic pathways, including glucose metabolism. The penetrance of the disease for WPW syndrome was complete, but the expression was variable. The described phenotype of this syndrome is similar to the autosomal recessive glycogen storage disease, Pompe disease. Given the function of the AMP-activated protein kinase and this similarity, the PRKAG2 syndrome is likely a cardiac-specific glycogenosis syndrome. This syndrome thus belongs to the group of genetic metabolic cardiomyopathies, rather than to the congenital primary arrhythmia syndromes. The annulus fibrosus, which normally insulates the ventricles from the atria, is thinned and disrupted by glycogen-filled myocytes, and these anomalous microscopic AV connections, rather than morphologically distinct BTs, appear to provide the anatomic substrate for ventricular preexcitation. Of note, certain mutations in PRKAG2 have been associated with nodoventricular BTs.
Another genetic form of WPW syndrome is associated with mutations in the bone morphogenetic protein-2 (BMP2) gene, which belongs to the class of transforming growth factor, The TGF-β-superfamily of proteins, and is involved in the development of the annulus fibrosus. This syndrome is characterized by variable cognitive deficits and dysmorphic features in addition to ventricular preexcitation.
Concealed Bypass Tracts
The true prevalence of concealed BTs is unknown because, unlike the situation with the WPW ECG pattern, these BTs are concealed on the surface ECG and are only expressed during AVRT; only symptomatic patients undergo EP testing. As noted, orthodromic AVRT accounts for approximately 95% of AVRTs and 35% of all paroxysmal SVTs, and 50% of the BTs that participate in orthodromic AVRT are concealed. SVTs using a concealed BT have no gender predilection and tend to occur more frequently in younger patients than in those with AVNRT; however, significant overlap exists. PJRT most often occurs in early childhood, although clinically asymptomatic patients presenting later in life are not uncommon.
Clinical Presentation
The majority of patients with preexcitation are asymptomatic and are discovered incidentally on an ECG obtained for unrelated reasons. When symptomatic arrhythmias occur in the WPW patient, the disorder is called the WPW syndrome. The two most common types of arrhythmias in the WPW syndrome are AVRT and AF. Patients with AVRT experience symptoms characteristic of paroxysmal SVT with abrupt onset and termination, including rapid and regular palpitations, chest pain, dyspnea, presyncope, and rarely, syncope. Episodes can last from seconds to several hours. Symptoms are usually mild and short-lived and terminate spontaneously or with vagal maneuvers. However, occasionally patients present with disabling symptoms, especially in the presence of structural heart disease.
AVRT, which in general is well tolerated by the patient when additional heart disease is absent, can deteriorate into AF; the latter can be a life-threatening arrhythmia if the BT has a short anterograde refractory period, resulting in very fast ventricular rates, with possible degeneration into VF and SCD. The incidence of SCD in patients with the WPW syndrome has been estimated to range from 0.15% to 0.39% over a 3- to 10-year follow-up. It is unusual for cardiac arrest to be the first symptomatic manifestation of WPW syndrome. Conversely, in about 50% of cardiac arrest cases in WPW patients, it is the first manifestation of WPW syndrome.
PJRT commonly presents as a frequently recurring or incessant tachycardia that is refractory to drug therapy and can lead to tachycardia-induced cardiomyopathy and heart failure symptoms.
Rarely, significant ventricular preexcitation during NSR can result in ventricular dysfunction secondary to dyssynchronous ventricular contraction. Improvement of left ventricular ejection fraction has been described after ablation of septal BTs in pediatric patients.
Initial Evaluation
History, physical examination, and 12-lead ECG constitute an appropriate initial evaluation. In patients with brief, self-terminating episodes of palpitations, an event recorder is the most effective way to obtain ECG documentation. Also, echocardiographic examination is recommended to exclude structural heart disease.
Several other noninvasive tests have been proposed as useful for evaluating symptomatic patients and risk-stratifying patients for SCD risk. However, the sensitivity and specificity of noninvasive testing have been shown to be limited. Invasive EP testing may be considered in patients with arrhythmias and those with a WPW ECG pattern when noninvasive testing does not lead to the conclusion that the anterograde ERP of the BT is relatively long. However, a strategy to perform an EP study for all asymptomatic patients with the WPW ECG pattern for the purpose of risk stratification is still controversial and not widely accepted.
Methods for Evaluation of Bypass Tract Refractory Period
Demonstration of Intermittent Preexcitation
Intermittent preexcitation has historically been thought to confer a lower risk of SCD than persistent preexcitation. Observation of intermittent loss of the preexcitation pattern on ambulatory monitoring or serial ECGs is generally correlated with a long BT anterograde ERP. The longer refractory period of the BT decreases the frequency of manifest preexcitation during NSR and is expected to lower the risk of mediating rapid preexcited ventricular activation during AF. Nevertheless, EP studies in symptomatic patients with intermittent preexcitation found that 10% to 24% can have BTs capable of conducting at rapid rates during AF (with anterograde ERP or shortest preexcited R-R interval less than 250 milliseconds), perhaps related to autonomic influences. However, similar findings have not yet been demonstrated in asymptomatic patients with intermittent preexcitation.
Intermittent preexcitation can be observed on ambulatory monitoring in up to 67% of patients. It is important, however, to distinguish intermittent preexcitation from inapparent preexcitation (see later) and from a bigeminal ventricular rhythm with a long coupling interval. Although intermittent preexcitation is a predictor of poor anterograde conduction through the BT, it has rarely been observed in some patients with cardiac arrest. Furthermore, the presence of intermittent preexcitation does not predict retrograde conduction properties of the BT nor preclude the development of AVRT.
Loss of Preexcitation During Exercise
Demonstration of a sudden loss of preexcitation (indicated by abrupt loss of the delta wave associated with prolongation of the PR interval and normalization of the QRS) during exercise is consistent with block in the BT and is consistent with a long BT ERP (greater than 300 milliseconds). Importantly, rapid AVN conduction during exercise can potentially mask persistent preexcitation. Therefore only abrupt and complete loss of preexcitation during exercise should be sought as a surrogate of a long anterograde ERP of the BT.
Loss of preexcitation during exercise is a good predictor that the patient is not at risk for VF even during sympathetic stimulation. However, the frequency of block in the BT during exercise is low (approximately 10% to 20%), and thus sensitivity of this test is poor. On the other hand, persistence of preexcitation during exercise stress has a sensitivity of 96% but a specificity of only 17% in predicting either a shortest preexcited R-R less than 250 milliseconds during AF or a BT ERP of less than 250 milliseconds (positive predictive value of 40% and negative predictive value of 88%).
Bypass Tract Conduction Block in Response to Antiarrhythmic Agents
When the administration of ajmaline (1 mg/kg IV over 3 minutes) or procainamide (10 mg/kg IV over 5 minutes) results in complete block of the BT during NSR, a long anterograde ERP (greater than 270 milliseconds) of the BT is likely. The shorter the BT ERP, the less likely it would be blocked by these drugs. Also, the amount of ajmaline required to block conduction over the BT correlates with the duration of the anterograde ERP of the BT. However, the incidence of BT block in response to these drugs is low and, although the occurrence of block predicts a long ERP of the BT, failure to produce block does not necessarily suggest a short ERP. Moreover, pharmacological testing is carried out at rest and therefore does not indicate what effect the drug will have on the BT ERP during sympathetic stimulation, such as exercise, emotion, anxiety, and recreational drug use. Importantly, the specificity of loss of preexcitation after administration of sodium blockers is poor compared to the shortest preexcited R-R intervals during inducible AF. Given these limitations, pharmacologic challenge is no longer routinely utilized.
Evaluation of Ventricular Response During Atrial Fibrillation
During spontaneous or induced AF, the propensity for rapid AV conduction can be judged by the interval between consecutively preexcited QRS complexes. A mean preexcited R-R interval greater than 250 milliseconds and a shortest preexcited R-R greater than 220 milliseconds predict low risk for SCD, with a negative predictive value of more than 95%; however, the positive predictive value is low (20%).
Response of Preexcitation to Transesophageal Atrial Stimulation
There is good correlation between the value of the anterograde ERP of the BT obtained during single-test programmed atrial stimulation and atrial pacing at increasing rates and the ventricular rate during AF. Programmed electrical stimulation of the atrium can be performed by the transesophageal route and the value of the anterograde ERP of the BT can be determined.
Electrophysiological Testing
Programmed atrial stimulation is used to evaluate the anterograde ERP of the BT. Because BT refractoriness shortens with decreasing pacing cycle length (PCL), the ERP should be determined at multiple PCLs (preferably ≤400 milliseconds). In addition, atrial stimulation should be performed close to the BT atrial insertion site to obviate the effect of intraatrial conduction delay. Incremental-rate atrial pacing is performed to determine the maximal rate at which 1:1 conduction over the BT occurs. Induction of AF should be performed to determine the average and the shortest R-R interval during preexcited AF. Atrial and ventricular stimulation is also performed to evaluate inducibility of AVRT as well as the number and location of BTs.
A shortest preexcited R-R interval less than 220 to 250 milliseconds during AF has been shown to be the best discriminator of those at risk of VF, with a high sensitivity (88% to 100%) and a high negative predictive value for identifying children and young adults with WPW syndrome at risk for VF. However, the positive predictive value is low (19% to 38%), largely due to the very low incidence of SCD in these patients. Also, a shortest preexcited R-R interval less than 250 milliseconds during AF has been noted in 20% to 26% of asymptomatic adults with a WPW pattern, and in up to 67% when isoproterenol is administered. Thus, although isoproterenol raises the sensitivity of invasive EP testing, it markedly reduces the specificity.
On the other hand, BT ERP less than 240 milliseconds appears to significantly correlate with only AVRT inducibility, but as an isolated variable, it is less predictive of life-threatening events and exhibits significant overlap between WPW patients with VF and those without VF. The presence of multiple BTs and the ability to induce sustained AVRT, especially when AVRT spontaneously degenerates into AF, have been proposed to predict risk of malignant ventricular arrhythmias. The lack of retrograde conduction over the BT appears to be at lower risk for SCD. However, the predictive value of these criteria remains limited and significant overlap exists.
Principles of Management
Acute Management
Symptomatic Patients With Concealed Bypass Tracts
Patients with orthodromic AVRT utilizing a concealed BT are treated in a similar fashion as those with paroxysmal SVT. Vagal maneuvers (including Valsalva and carotid sinus massage) are the first-line intervention for acute conversion of the tachycardia; though the overall success rate is limited (approximately 28%). If SVT persists, adenosine is recommended, and it offers a success rate of 90% to 95%. For refractory tachycardia, IV diltiazem, verapamil, or beta-blockers can terminate orthodromic AVRT in the majority of patients. Synchronized cardioversion is recommended for hemodynamically unstable patients and for those refractory or intolerant to drug therapy ( Fig. 18.7 ).

Symptomatic Patients With Manifest Bypass Tracts
In patients with manifest preexcitation during NSR presenting with AVRT (orthodromic or antidromic), vagal maneuvers are the first-line intervention for tachycardia termination (see Fig. 18.7 ). For persistent SVT, adenosine is recommended. Importantly, adenosine should be used with caution because it can induce AF with a rapid ventricular rate in the presence of an anterogradely conducting BT. This is unusual and should not be viewed as a contraindication to adenosine use, but one should be prepared for emergency cardioversion before administering adenosine to SVT patients.
For refractory AVRT, IV diltiazem, verapamil, or beta-blockers can be considered to block conduction in the AVN, which represents either the retrograde or anterograde limb in the AVRT circuit. AVN blocking drugs, however, are ineffective in patients with preexcited AVRT that utilizes two separate BTs for anterograde and retrograde conduction. Drug treatment directed at the BT (ibutilide, procainamide, flecainide) may also be considered. When drug therapy fails or hemodynamic instability is present, electrical cardioversion should be considered. It is important to note that IV diltiazem and verapamil can result in hemodynamic collapse if the AVRT does not terminate or AF is induced with rapid conduction over the BT degenerating to VF, and one must be prepared for immediate electrical cardioversion if that occurs (see later).
Preexcited Atrial Fibrillation
In patients with AF or AFL, ventricular preexcitation, and rapid ventricular response, prompt direct-current cardioversion is recommended, especially when hemodynamic compromise is present. IV procainamide or ibutilide to restore NSR or slow the ventricular rate may be considered in hemodynamically stable patients. Both drugs slow BT conduction.
Importantly, drugs that preferentially slow AVN conduction without prolonging BT refractoriness (such as verapamil, diltiazem, beta-blockers, adenosine, oral or IV digoxin, and IV amiodarone) can accelerate the ventricular rate and potentially precipitate hemodynamic collapse and VF in high-risk patients. Several mechanisms are probably involved; hypotension produced by some of those medications is followed by a sympathetic discharge that enhances BT conduction. Furthermore, slowing or blocking conduction through the AVN prevents competitive concealed retrograde conduction into the BT by normally conducted beats and, as a result, can potentially enhance conduction over the BT. In addition, the rapid and irregular ventricular rate, hypotension, and sympathetic discharge probably result in fractionation of the ventricular wavefront and VF. Also, digoxin increases the ventricular rate by shortening BT refractoriness. Lidocaine, for reasons that are unclear, has also been associated with degeneration of AF into VF. It is occasionally used in patients with WPW who have a wide QRS complex tachycardia that might be misinterpreted as VT. Unlike the IV route of administration, chronic oral amiodarone therapy can slow or block BT conduction.
Chronic Management
Symptomatic Patients With Concealed Bypass Tracts
Catheter ablation is considered first-line therapy (class I) for patients with paroxysmal SVT involving a concealed BT (i.e., orthodromic AVRT). However, because concealed BTs are not associated with an increased risk of SCD in these patients, catheter ablation can be presented as one of a number of potential therapeutic approaches, including pharmacological therapy and clinical follow-up alone. When pharmacological therapy is selected for patients with concealed BTs, it is reasonable to consider a trial of beta-blocker therapy, diltiazem, or verapamil ( Fig. 18.8 ). These agents are effective for preventing recurrent tachycardia in approximately 50% of patients. Antiarrhythmic agents may be considered for patients with refractory tachycardia; however, the risk and benefits of these drugs should be carefully considered.

Symptomatic Patients With Manifest Bypass Tracts
Catheter ablation is considered the treatment of choice for patients with WPW syndrome—that is, patients with manifest preexcitation along with documented arrhythmias (antidromic or orthodromic AVRT or preexcited AF) or symptoms consistent with cardiac arrhythmias. Catheter ablation is curative in more than 95% of patients with a relatively low complication rate (about 3%), and it also obviates the unwanted side effects of pharmacological therapy.
For patients with WPW syndrome who are not candidates for, or prefer not to undergo catheter ablation, antiarrhythmic drugs to block BT conduction are considered (see Fig. 18.8 ). Class IC agents such as flecainide and propafenone (in patients without structural heart disease or ischemic heart disease), and class III drugs, such as sotalol and dofetilide, may be considered. Oral amiodarone is a last resort therapy, given the associated risks of long-term therapy. In general, antiarrhythmic drug therapy can offer symptomatic improvement in up to 90% of patients, although complete disappearance of symptoms is observed in only 30%.
Chronic oral beta-blocker, verapamil, and diltiazem may be used for the treatment of patients with WPW syndrome, particularly if their BT has been demonstrated to be incapable of rapid anterograde conduction. However, these agents must be used with caution and after a discussion with the patient concerning the potential risk of rapid conduction over the BT if AF develops. Digoxin, on the other hand, should be avoided because it can shorten the refractory period of the BT and, hence, is potentially harmful in patients with manifest BTs.
Asymptomatic Patients With Manifest Bypass Tracts
Asymptomatic young WPW patients have about 30% risk of becoming symptomatic, and a very small, but definite risk of life-threatening arrhythmias and SCD. Therefore, in the recent Pediatric and Congenital Electrophysiology Society (PACES) and the Heart Rhythm Society (HRS) expert consensus document on treatment of asymptomatic young WPW subjects, risk stratification is recommended to identify a potential subgroup of patients with BTs with “high-risk” properties that may confer an increased risk for lethal cardiac arrhythmias and in whom the risk-to-benefit ratio favors prophylactic ablation ( Fig. 18.9 ).
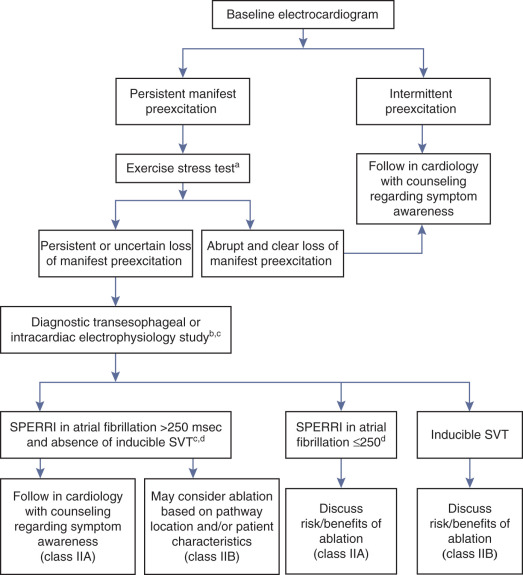
Initial risk stratification utilizes noninvasive testing (e.g., Holter monitoring, exercise stress testing) to ascertain true loss of preexcitation at physiological heart rates. Complete and abrupt loss of preexcitation during exercise testing or intermittent loss of preexcitation during ECG or ambulatory monitoring, indicate long anterograde ERP of the BT and help identify patients at low risk of rapid conduction over the BT and SCD ( Box 18.1 ).
- •
Intermittent loss of the preexcitation pattern on ambulatory monitoring or serial electrocardiograms
- •
Abrupt and complete loss of preexcitation during exercise
- •
Loss of preexcitation after administration of sodium blockers
- •
Mean preexcited R-R interval >250 msec and shortest preexcited R-R >220 msec during atrial fibrillation
- •
Anterograde effective refractory period of the bypass tract <240 msec
Inability to clearly demonstrate absolute loss of ventricular preexcitation on noninvasive testing warrants consideration for transesophageal or intracardiac EP testing. However, the benefits and risk of invasive risk stratification should be based on individual considerations such as age, gender, occupation, and athletic involvement, and should be thoroughly discussed with the patient or, in the case of a child, with the parents. Given the low risk associated with invasive EP testing (0.1% to 1%), it is reasonable to consider this approach for risk stratification in asymptomatic WPW patients. Several EP findings help identify high-risk patients who may benefit from catheter ablation, including: (1) shortest preexcited R-R interval less than 250 milliseconds during induced AF; (2) the presence of multiple BTs; (3) spontaneous degeneration of induced AVRT into preexcited AF; and (4) BT anterograde ERP less than 240 milliseconds. The critical obligatory condition for VF is the presence of a short anterograde functional refractory period of the BT, which is best reflected in the shortest R-R interval between preexcited beats in AF. Although the ability to induce sustained AVRT has been considered by some as a potential risk factor, this has not been supported by recent studies.
The role of isoproterenol challenge during EP testing has not yet been clearly defined. Isoproterenol administration can significantly shorten the shortest preexcited R-R interval and, as a result, increase the proportion of asymptomatic patients in the “high-risk” category. Thus some investigators suggested the use of a more stringent threshold for the shortest preexcited R-R interval (≤220 milliseconds) instead of the threshold adopted by the 2012 PACES/HRS Guidelines (≤250 milliseconds) for definition of “high-risk” BTs when EP testing is performed under the influence of isoproterenol.
In low-risk patients, as determined by noninvasive or invasive testing, it is appropriate to pursue a strategy of follow-up with ECGs and reevaluation at selected intervals with a high degree of suspicion for new arrhythmia symptoms. This strategy should incorporate patient education about the potential risks associated with preexcitation and the symptoms of arrhythmias that should prompt them to seek medical attention. It is also advisable to give the patient a copy of his or her ECG and a short note about the fact that the WPW pattern is present to help prevent the misdiagnosis of MI and to explain the basis of cardiac arrhythmias in case they develop later. Importantly, WPW patients most susceptible to SCD are symptomatic. Thus the evolution of the clinical status from an asymptomatic state to symptoms (e.g., syncope or palpitations) likely portends a higher risk for SCD. Once WPW patients become symptomatic, catheter ablation may be considered, regardless of the prior risk assessment.
In patients with high-risk BT characteristics, prophylactic catheter ablation is reasonable. A combination of inducible AVRT and short R-R interval during preexcited AF provides the best indication for ablation. Catheter ablation of the BT, regardless of BT characteristics, is also reasonable in asymptomatic patients with high-risk occupations (e.g., school bus drivers, police, and pilots), and is probably reasonable in: (1) patients involved in moderate- to high-intensity competitive sports; (2) patients with structural heart disease; (3) patients with ventricular dysfunction secondary to dyssynchronous contractions; and (4) patients with BT locations associated with lower risk of procedural complications (such as AV block or coronary artery injury), which can counterbalance the potential benefit of ablation. However, because knowledge about the success and complication rates plays a major role in decision-making, the physician must consider his or her own success and complication rates for ablation of the specific location of the BT identified, and that information should be made available to the patient.
It is important to recognize that the majority of adult patients with asymptomatic preexcitation have a benign course with few clinically significant arrhythmic events occurring over time; the risk of SCD is low, is seen mainly in children, and is rarely the initial clinical manifestation. Hence, observation without further evaluation or treatment remains a reasonable option in these patients, even when noninvasive low-risk markers are not evident. The key is a clear understanding by the patient of the relative merits of each strategy. The well-informed patient needs to choose between a very small risk of potentially life-threatening arrhythmia over a long period of time and a one-time small procedural risk associated with EP testing and catheter ablation. Certain patients such as athletes and those in higher risk occupations will generally choose ablation. Others, especially patients older than 30 years, may prefer the small risk of a conservative strategy.
It is important to note that both invasive and noninvasive EP markers, despite their high sensitivity and negative predictive value, lack specificity for identifying patients at risk of life-threatening ventricular arrhythmias, largely due to the very low incidence of SCD. The great majority of individuals with WPW, even with a shortest preexcited R-R interval less than 250 milliseconds during AF, will not experience SCD; thus the positive value of predicting SCD remains very low. Therefore the management of asymptomatic patients with a WPW pattern remains controversial.
The ability of noninvasive risk stratification to identify low-risk patients is low (less than 20%). Hence, according to the 2012 PACES/HRS Guidelines, invasive EP evaluation would be recommended for the majority of young asymptomatic WPW patients, and the majority of those would undergo catheter ablation. A recent report retrospectively examined the consequences of following the published guidelines in 85 asymptomatic patients (less than 18 years old) with ventricular preexcitation ECG pattern persisting at peak exercise, to assess the outcomes of invasive risk stratification applying current guidelines. Approximately 38% of the patients exhibited adverse BT properties at EP study, fulfilling either the class IIA indication (shortest preexcited R-R interval less than 250 milliseconds during AF) or class IIB indication (AVRT inducibility) for catheter ablation. The use of isoproterenol infusion during EP testing shifted an additional 36% of those tested into one of these two indication classes. About 69% of young patients subjected to risk stratification underwent BT ablation as a result of the evaluated BT properties or patient/parental decision.
Although complications of a diagnostic EP study are generally minor and not life-threatening, the risks associated with an ablation procedure are likely at least similar to the risk of SCD in asymptomatic WPW patients. In three large series, procedure-related complications occurred in 1.8% to 8.2% of cases, and death as a consequence of ablation occurred in 0.07% to 0.19%. If routine EP testing were to be performed in the majority of asymptomatic WPW patients, many patients would proceed immediately to catheter ablation and, in others, there would be a strong temptation to ablate when catheters are in place (regardless of predicted SCD risk), especially given the fact that the criteria for ablation usually will not be black or white. This greatly increases the risk to the patient, which can potentially nullify the benefit of elimination of SCD risk achieved by BT ablation. A recent study using decision analysis software to construct a risk-benefit decision tree for a target population of 20- to 40-year-old asymptomatic patients with WPW, found the decision to ablate resulted in a reduction of 10-year mortality risk of 8.8 patients for 1000 patients. The study suggested that it is necessary to treat 112 asymptomatic patients with WPW to save one life over 10 years.
Finally, the physician and the patient must have a shared understanding of the value of invasive EP study for risk stratification, rather than as a therapeutic tool. These issues have to be resolved before proceeding with invasive measures, and the risks and benefits of proceeding with ablation of BT found not to have high-risk characteristics should be discussed thoroughly with patients in advance of the EP procedure.
Wolff-Parkinson-White Patients and Sports Participation
WPW syndrome accounts for approximately 1% of deaths in athletes. Although many of the cases of SCD with WPW are associated with exercise, training does not alter the EP properties in WPW.
Catheter ablation is the treatment of choice for symptomatic WPW patients (whether or not engaged in athletics). For asymptomatic WPW patients engaged in moderate- to high-level competitive sports, noninvasive and, if needed, invasive risk stratification is advisable. For those with high-risk BT characteristics, ablation of the BT is recommended before clearance for competitive sports because of risk for life-threatening arrhythmias. In low-risk patients, as determined by noninvasive or invasive testing, BT ablation may still be appropriate, but competitive sports may be allowed without ablation, especially when BT ablation confers an unacceptable potential risk (e.g., AV block in the setting of midseptal or superoparaseptal BTs).
Electrocardiographic Features
Electrocardiography of Preexcitation
Anterogradely conducting AV BTs produce the classic WPW ECG pattern characterized by a fusion between conduction via the BT and the normal AVN-HPS: (1) short PR (P-delta) interval (less than 120 milliseconds); (2) slurred upstroke of the QRS (delta wave); and (3) wide QRS (>120 milliseconds) (see Figs. 18.1 and 18.2 ).
The degree of preexcitation depends on several factors, including conduction time over the AVN-HPS, conduction time from the sinus node to the atrial insertion site of the BT (which depends on the distance, conduction, and refractoriness of the intervening atrial tissue), and conduction time through the BT (which depends on the length, thickness, and conduction properties of the BT).
Pharmacological and/or physiological maneuvers that alter AVN conduction (e.g., carotid sinus massage, Valsalva maneuvers, adenosine, beta-blockers) can be used to alter the degree of preexcitation, thereby confirming the diagnosis of the presence of an anterogradely conducting AV BT.
The ECG pattern displayed by some patients with WPW syndrome can simulate the pattern found in other cardiac conditions and can alter the pattern seen in the presence of other cardiac disease. A negative delta wave (presenting as a Q wave) can mimic a myocardial infarction (MI) pattern. Conversely, a positive delta wave can mask the presence of a previous MI. Intermittent WPW can also be mistaken for frequent premature ventricular complexes (PVCs) ( Fig. 18.10 ). If the WPW pattern persists for several beats, the rhythm can be misdiagnosed as an accelerated idioventricular rhythm or, if sufficiently rapid, VT. The WPW pattern is occasionally seen on alternate beats and may suggest ventricular bigeminy. An alternating WPW and normal pattern can occasionally suggest electrical alternans. On the other hand, late-coupled PVCs ( eFig. 18.2 ) and ventricular pacing ( eFig. 18.3 ) with inapparent pacing artifacts can occasionally mimic ventricular preexcitation.



Inapparent Versus Intermittent Preexcitation
Inapparent preexcitation.
With inapparent preexcitation, preexcitation is absent on the surface ECG despite the presence of an anterogradely conducting AV BT because conduction over the AVN-HPS reaches the ventricle faster than that over the BT. In this setting, the PR interval is shorter than the P-delta interval would be if preexcitation were present. Therefore the transition from manifest to inapparent preexcitation is characterized by normalization of the QRS in conjunction with shortening of the PR interval, reflecting the now better AVN-HPS conduction.
Inapparent preexcitation is usually caused by: (1) enhanced AVN conduction, so that it is faster than conduction over the BT; (2) prolonged intraatrial conduction from the site of atrial stimulation to the atrial insertion site of the BT (most often left lateral), favoring anterograde conduction and depolarization of the ventricle over the AVN-HPS; or (3) prolonged conduction over the BT, so that it is slower than AVN-HPS conduction.
Intermittent preexcitation.
Intermittent preexcitation is defined as the presence and absence of preexcitation on serial ECGs or ambulatory cardiac monitoring ( Fig. 18.11 ). True intermittent preexcitation is characterized by an abrupt loss of the delta wave (regardless of how fast or slow AVN conduction is), with prolongation (normalization) of the PR interval (reflecting the loss of the faster BT conduction, and the subsequent conduction over the slower AVN), and normalization of the QRS in the absence of any significant change in heart rate.

The mechanism of intermittent preexcitation is poorly understood, but is likely related to the BT refractory period and cellular connectivity within the BT. Potential mechanisms include (1) phase 3 (i.e., tachycardia-dependent) or phase 4 (i.e., bradycardia-dependent) block in the BT ( see Chapter 10 ); (2) anterograde or retrograde concealed conduction produced by PVCs, premature atrial complexes (PACs), or atrial arrhythmias; (3) BTs with a long ERP and the gap phenomenon in response to PACs; and (4) BTs with a long ERP and supernormal conduction.
Preexcitation alternans is a form of intermittent preexcitation in which a QRS complex manifesting a delta wave alternates with a normal QRS complex (see Fig. 18.10 ). Concertina preexcitation is another form of intermittent preexcitation in which the PR intervals and QRS complex durations show a cyclic pattern; that is, preexcitation becomes progressively more prominent over a number of QRS complex cycles followed by a gradual diminution in the degree of preexcitation over several QRS cycles, despite a fairly constant heart rate.
Differentiation between intermittent preexcitation and inapparent preexcitation on an ECG showing QRS complexes with and without preexcitation can be achieved by comparing the P-delta interval during preexcitation and the PR interval when preexcitation is absent. Loss of preexcitation associated with a PR interval longer than the P-delta interval is consistent with intermittent preexcitation (see Fig. 18.11 ), whereas loss of preexcitation associated with a PR interval shorter than the P-delta interval is consistent with inapparent preexcitation. Furthermore, maneuvers that slow AVN conduction (e.g., carotid sinus massage, AVN blockers) would unmask inapparent preexcitation but would not affect intermittent preexcitation.
Supraventricular Tachyarrhythmias Associated With Wolff-Parkinson-White Syndrome
Orthodromic Atrioventricular Reentrant Tachycardia
The ECG during orthodromic AVRT shows retrograde P waves inscribed within the ST-T wave segment with an RP interval that is usually less than half of the tachycardia R-R interval (i.e., RP interval <PR interval) ( Fig. 18.12 ). The RP interval remains constant, regardless of the tachycardia cycle length (TCL), because it reflects nondecremental retrograde conduction over the BT. QRS morphology during orthodromic AVRT is generally normal and not preexcited, even when preexcitation is present during NSR (see Fig. 18.5 ). Functional bundle branch block (BBB) can be observed frequently during orthodromic AVRT at fast rates (see Fig. 18.12 ). The presence of BBB during SVT in a young person (less than 40 years) should raise the suspicion of orthodromic AVRT incorporating a BT ipsilateral to the blocked bundle, because the longer conduction time through the involved ventricle engendered by the BBB facilitates orthodromic reentry by enabling all portions of the circuit enough time to recover excitability from the prior cycle. This is particularly true with left bundle branch block (LBBB), which is very uncommon in younger patients.

Orthodromic AVRT tends to be a rapid tachycardia, with rates ranging from 150 to more than 250 beats/min, generally faster in younger people. A beat-to-beat oscillation in QRS amplitude (QRS alternans) is present in up to 38% of cases and is most commonly seen when the rate is very rapid ( Fig. 18.13 ). The mechanism for QRS alternans is not clear but may partly result from oscillations in the relative refractory period of the distal portions of the HPS.

Ischemic-appearing ST segment depression also can occur during orthodromic AVRT, even in young individuals who are unlikely to have coronary artery disease. An association has been observed between repolarization changes (ST segment depression or T wave inversion) and the underlying mechanism of the tachycardia because such changes are more common in orthodromic AVRT than AVNRT (57% vs. 25%). Several factors can contribute to ST segment depression in these arrhythmias, including changes in autonomic tone, intraventricular conduction disturbances, a longer VA interval, and a retrograde P wave of longer duration that overlaps into the ST segment. The location of the ST segment changes can vary with the location of the BT; ST segment depression in leads V3 to V6 is almost invariably seen with a left lateral BT, whereas ST segment depression and a negative T wave in the inferior leads is associated with a posteroseptal or posterior BT. A negative or notched T wave in leads V2 or V3 with a positive retrograde P wave in at least two inferior leads suggests an anteroseptal BT. However, ST segment depression occurring during orthodromic AVRT in an older patient mandates consideration of possible coexisting ischemic heart disease.
Antidromic Atrioventricular Reentrant Tachycardia
Antidromic AVRT is characterized by a wide (fully preexcited) QRS complex, usually regular R-R intervals, and ventricular rates of up to 250 beats/min (see Fig. 18.5 ). The width of the preexcited QRS complex and the amplitude of the ST-T wave segment usually obscure the retrograde P wave on the surface ECG. When the P waves can be identified, they are inscribed within the ST-T wave segment with an RP interval that may be more than half of the tachycardia R-R interval because retrograde conduction occurs slowly via the AVN-HPS. The PR (P-delta) interval remains constant, regardless of the TCL, because it represents nondecremental anterograde conduction over the BT.
Permanent Junctional Reciprocating Tachycardia
PJRT tends to be incessant, stopping and starting spontaneously every few beats without initiating PACs or PVCs. The heart rate is usually between 120 and 200 beats/min and the QRS duration is generally normal. Slow retrograde conduction over the BT causes the RP interval during PJRT to be long, usually more than half of the tachycardia R-R interval ( Fig. 18.14 ). The P waves resulting from retrograde conduction are easily seen on the ECG and are inverted in leads II, III, aVF, and V3 to V6.

Atrioventricular Nodal Reentrant Tachycardia and Atrial Tachycardia
Both AVNRT and AT can be associated with partially or fully preexcited QRS complex secondary to anterograde conduction to the ventricles over the bystander BT. When AVNRT occurs in the WPW syndrome, the arrhythmia can be difficult to distinguish from orthodromic AVRT without EP testing.
Atrial Fibrillation
There are several characteristic findings on the ECG in patients with AF conducting over a BT, so-called preexcited AF. The rhythm is irregularly irregular, and can be associated with very rapid ventricular response caused by the nondecremental anterograde AV conduction over the BT ( Fig. 18.15 ). However, a sustained rapid ventricular rate of more than 180 to 200 beats/min will often create R-R intervals that appear to be regular when the ECG is recorded at 25 mm/s. Although the QRS complexes are conducted aberrantly, resembling those during preexcited NSR, their duration can be variable and they can become normalized. This is not related to the R-R interval (i.e., it is not a rate-related phenomenon), but rather is related to the variable relationship between conduction over the BT and AVN-HPS. Preexcited and normal QRS complexes often appear “clumped.” This can result from concealed retrograde conduction into the BT or the AVN ( Fig. 18.16 ).


The QRS complex during preexcitation is a fusion between the impulse that preexcites the ventricles caused by rapid conduction through a BT and the impulse that takes the usual route through the AVN. The number of impulses that can be transmitted through the BT and the amount of preexcitation depend on the refractoriness of the BT and AVN. The shorter the anterograde ERP of the BT, the more rapid is the anterograde impulse conduction over the BT and, because of more preexcitation, the wider the QRS complexes. Patients who have a BT with a very short ERP and rapid ventricular rates represent the group at greatest risk for development of VF.
Anterograde block in the BT during AF abolishes retrograde concealment into the AVN, which in turn allows the AVN to recover its excitability and conduct anterogradely. In turn, these conducted impulses through the AVN can result in retrograde concealment into the BT, causing anterograde block of the BT and, thereby, slowing the ventricular rate.
Atrial Flutter
AFL, like AF, can conduct anterogradely via a BT resulting in a preexcited tachycardia. Depending on the various refractory periods of the normal and pathological AV conduction pathways, AFL can potentially conduct 1:1 to the ventricles during a preexcited tachycardia, making the arrhythmia difficult to distinguish from VT ( see Fig. 12.10 ).
Electrocardiographic Localization of the Bypass Tract
Careful analysis of the preexcitation pattern during NSR can potentially allow an accurate approximation of the location of the BT. This provides the electrophysiologist with important information that can guide patient counseling regarding the risks and benefits of ablation. In particular, it provides some guidance about the proximity of the BT to the normal conduction system and the subsequent risk of AV block associated with an ablation attempt, as well as the need for left heart catheterization and atrial septal puncture and their potential complications. In addition, it can help in planning the ablation procedure, such as the use of cryoablation for septal BTs or the need for special equipment for atrial septal puncture for left-sided BTs.
Localization Using the Delta Wave
Delta wave morphology reflects the ventricular insertion site of the BT and, hence, is helpful in approximating the BT location, especially when maximal preexcitation is present. However, during NSR, only partial ventricular preexcitation is usually observed, which limits the accuracy of BT localization based on the surface ECG. In addition, the degree of preexcitation can vary in an individual, depending on heart rate, autonomic tone, and AVN function, as well as the location and EP characteristics of the BT. Therefore it is important first to assess the degree of preexcitation visible throughout the entire ECG and then use only delta wave polarity for localization (the first 20 to 60 milliseconds of the QRS in most cases, unless fully preexcited) rather than the overall QRS polarity, which may vary from each other.
Several algorithms have been developed to predict the anatomic location of manifest BTs based on delta wave polarity on the surface ECG ( Box 18.2 ; Figs. 18.17–18.20 ). Although these algorithms facilitate prediction of the location of a BT, they are inherently limited by biological variability in anatomy (e.g., rotation of the heart within the thorax), variable degree of preexcitation and QRS fusion, the presence of more than one manifest BT, intrinsic ECG abnormalities (such as prior MI and ventricular hypertrophy), patient body habitus, and technical variability in ECG acquisition and electrode positioning. The accuracy of these algorithms in more recent studies has not reached the accuracy previously reported by their designers. Therefore these algorithms should be regarded as an orientation rather than a precise localization tool. No single published algorithm offers extremely high sensitivity and specificity for all BT locations. The algorithms appear most accurate in predicting left free-wall BT locations and least accurate in predicting midseptal or right anteroseptal BT locations. Hence, it may be more realistic to initially identify a general area in which the BT is located, and then to apply more subtle criteria from one or more of the algorithms to attempt a more precise localization. This can be achieved by applying some basic rules while maintaining a mental 3-D representation of the mitral and tricuspid annuli and their anatomical relationships to adjacent structures as they lie within the chest (i.e., “attitudinally correct orientation”) while analyzing the ECG and predicting morphology of the preexcited QRS.
a A, algorithm of Chiang and colleagues; B, algorithm of Fitzpatrick and colleagues; C, algorithm of Xie and colleagues.
Left Lateral/Left Anterolateral BTs
- A.
R/S ≥1 in V2 and positive delta in III, or R/S <1 in V2 and positive delta in III and V1
- B.
RS transition ≤V1 and >2 positive delta in the inferior leads or S > R in aVL
- C.
QS or QR morphology in aVL and no negative QRS in III and V1
Left Posterior/Left Posterolateral BTs
- A.
R/S ≥1 in V1 and V2, no positive delta in III and positive delta in V1
- B.
R/S transition ≤V1, no ≥2 positive delta in the inferior leads, no S > R in aVL, in lead I R <(S 0.8 mV), and the sum of the inferior delta is not negative
- C.
Positive QRS in aVL and an equiphasic QRS or positive in V1 and no negative QRS in III
Left Posteroseptal BTs
- A.
R/S ≥1 in V2, no positive delta in III, and positive delta and R/S <1 in V1
- B.
R/S transition ≤V1, no ≥2 positive delta in the inferior leads, no S > R in aVL, in lead I R <(S 0.8 mV), and the sum of the inferior delta is negative
- C.
Negative QRS in III, V1, and aVF, tallest precordial R wave in V2–V4 and R wave width in V1 >0.06 msec
Midseptal BTs
- A.
R/S <1 in V2, no positive delta in III, and negative delta in V1
- B.
R/S transition between V2 and V3 or between V3 and V4 but the delta amplitude in lead II ≥1.0 mV (septal location), and the sum of delta polarities in inferior leads is −1, 0, or +1 mV
- C.
Negative QRS in leads III, V1, and aVF, tallest precordial R in leads V2–V4 and R wave width in V1 <0.06 msec
Right Posteroseptal BTs
- A.
R/S ≥1 in V2 and no positive delta in III and V1
- B.
The sum of delta polarities in inferior leads is less than or equal to −2 mV
- C.
Negative QRS in leads III, V1, and aVF and tallest precordial R in V5 or V6
Right Posterior/Right Posterolateral BTs
- A.
R/S <1 in V2, no positive delta in III and no negative delta in V1, and negative delta polarity in aVF
- B.
R/S transition between V3 and V4 with delta amplitude in lead II <1.0 mV or ≥V4 and the delta axis is <0 degrees and the R in lead III is ≤0 mV
- C.
Positive QRS in III, negative in V1, and RS morphology in aVL
Right Lateral/Right Anterolateral BTs
- A.
R/S <1 in V2, no positive delta in III and no negative delta in V1 and negative or biphasic delta in aVF
- B.
R/S transition between leads V3 and V4 with delta amplitude in lead II <1.0 mV or >V4 and the delta axis is <0 degrees and the R amplitude in lead III is <0 mV
- C.
Positive QRS in leads aVL and III and negative in V1
Right Anterior/Right Superoparaseptal BTs
- A.
R/S <1 in V2, positive delta in III and no positive delta in V1
- B.
The sum of delta polarities in inferior leads is ≥2
- C.
Positive QRS in aVF and negative in leads III and V1
BT, Bypass tract; R/S, R-S wave ratio.




Of note, the mere presence and the degree of preexcitation can sometimes help in predicting the location of the BT. Posteroseptal and right-sided BTs tend to be associated with a prominent degree of preexcitation because of the proximity to the sinus node, whereas left lateral BTs are often associated with subtle preexcitation. Nevertheless, when preexcitation is prominent, the accuracy of surface ECG localization of manifest BTs tends to be higher for the diagnosis of left free-wall BTs than for BTs in other locations.
On the surface ECG, analysis of delta wave polarity and amplitude progression in precordial leads, and frontal plane horizontal and vertical axes, are valuable for predicting the site of origin of manifest BTs ( Fig. 18.21 ).

Precordial transition.
Lead V1 is a unipolar lead positioned at the right anterior chest wall. Therefore, as the BT location shifts progressively more to the left or posteriorly, the precordial transition (i.e., the first precordial lead where the R wave amplitude exceeds the S wave amplitude) becomes sequentially earlier, thereby transforming the precordial preexcited QRS morphology from a late transition LBBB pattern of the preexcited QRS to a positively concordant right bundle branch block (RBBB) pattern. Hence, left-sided BTs exhibit positive delta waves in lead V1, while right-sided BTs exhibit negative delta waves. The farther the BT is to the left or posteriorly on the mitral annulus, the larger the positive delta wave, and the farther the BT is to the right along the tricuspid annulus, the deeper the negative delta wave is in lead V1.
Frontal plane horizontal axis.
Lead I primarily reflects the horizontal axis. BTs closer to the left axilla will produce a deeply negative complex in lead I (i.e., rightward axis). Conversely, BTs closer to the right axilla are strongly positive in lead I (i.e., leftward axis). Leads II/aVL and III/aVR also have net leftward and rightward vectors, respectively. Hence, as the BT location moves progressively to the left (e.g., from anterior to lateral mitral annulus), the delta wave assumes progressively less positive/more negative deflection in lead I, a taller R wave in lead III than in lead II, and a larger S wave in lead aVL compared to lead aVR. Opposite changes are expected as the BT location moves progressively to the right (from anteromedial to lateral tricuspid annulus).
Frontal plane vertical axis.
The inferior leads (II, III, and aVF) reflect the vertical axis. Therefore BTs located at the superior aspect of the tricuspid or mitral annulus exhibit positive deflections in the inferior leads (i.e., vertical axis). The magnitude of the inferiorly directed vector diminishes as the site of origin shifts from superior to inferior regions of either annulus.
Left vs. right free-wall BTs.
Delta wave polarity in right precordial leads is most helpful in distinguishing between right- and left-sided free-wall BTs. Ventricular activation originating from the right ventricle (RV) (as mediated by right-sided BTs) is expected to produce a predominantly negative deflection in right precordial leads, whereas more posterior or left-sided sites of origin produce more positive deflections. Hence, an R/S ratio greater than 1 or a dominant R wave in lead V1 is consistent with left-sided BTs, whereas R/S transition after lead V2 suggests right-sided BTs. When the R/S transition is at lead V2 or between leads V1 and V2, the R/S wave in the left lateral limb leads (I and aVL) favors right-sided BTs, otherwise left-sided BTs are likely.
Left-sided BTs.
All left free-wall BTs exhibit positive delta wave polarity in the right precordial leads, an R/S ratio greater than 1, or a dominant R wave in lead V1. Because of its superior location in the LV, BTs originating from the anterior mitral annulus exhibit larger R wave amplitudes in the inferior leads. As the BT location shifts progressively more laterally, R wave amplitude becomes progressively larger in lead III and smaller in lead II. The delta wave amplitude ratio in leads III/II is greater than 1 for posterolateral BTs and less than 1 for anterolateral BTs. BTs located at the inferior aspects of the mitral annulus exhibit negative delta waves in the inferior leads. Furthermore, BT locations at the lateral aspect of the mitral annulus produce a more negative delta wave in the left lateral limb leads (aVL and I) than anterior or posterior annular locations. In fact, a negative delta wave in the left lateral leads (aVL, I, and V6) is pathognomonic of a left lateral BT.
Right-sided BTs.
BTs arising from the tricuspid annulus demonstrate negative delta wave polarity in the right precordial leads and positive polarity in leads V5 and V6. As the BT location moves from medial/septal to lateral locations along the tricuspid annulus, the precordial transition becomes later (at lead V3 in septal locations, and after lead V3 in free-wall locations) and the negative delta wave becomes deeper in anterior precordial leads (V1 to V3). Right-sided BTs typically display a large positive delta wave in the left lateral limb leads (aVL and I), but a predominantly negative delta wave in lead aVR. Delta wave polarity in the inferior leads is positive in anterior annular BTs. As the BT location moves laterally and inferiorly, the delta wave becomes more negative in the inferior leads. Positive delta waves in leads II and aVF are consistent with anteroseptal locations, whereas negative delta waves in the inferior leads suggest posteroseptal locations. Flat or biphasic delta waves suggest midseptal locations.
Anteroseptal BTs.
Right superoparaseptal (anteroseptal) BTs connect the RA and RV free walls at the anteromedial aspect of the tricuspid annulus. Because of its relatively superior and anterior ventricular insertion compared to the rest of the ventricular mass, these BTs exhibit positive delta waves in the inferior leads (II, III, and aVF). In addition, delta waves are often negative in leads V1 and V2, but slightly positive (R/S < 1) delta waves also are observed. Similar to right free-wall BTs, delta waves are typically positive in leads I and aVL and negative in lead aVR.
Midseptal BTs.
The delta wave is positive in lead II, predominantly negative in lead III, and negative or isoelectric in lead aVF. The delta wave in lead V1 is usually negative and R/S transition occurs after lead V2 (usually between leads V2 and V3). However, variations are not infrequent, especially in the inferior leads and in V2. Midseptal BTs can be differentiated from superoparaseptal and para-Hisian BTs by a negative delta wave in lead III, a biphasic delta wave in lead aVF, and a larger R/S ratio in lead V2, likely due to the posterior location of the AV septum compared to the anterior tricuspid annulus.
Posteroseptal BTs.
Posteroseptal BTs are characterized by a deeply negative delta wave in lead III. The delta wave is often negative in lead aVF, especially for right posteroseptal BT locations. A negative delta wave in lead II predicts an epicardial location of the posteroseptal BT. The delta wave in lead V1 can be negative, isoelectric, or positive. Lead V2 typically displays a positive delta wave, with the R wave larger than the S wave. A negative delta wave polarity in lead V1 with abrupt transition to positive polarity (with R/S ratio greater than 1) in lead V2 favors a right-sided location of the posteroseptal BT, whereas left posteroseptal BTs are often associated with biphasic or positive delta wave polarity in leads V1 and V2. An R/S ratio greater than 1 in lead V1 is a more accurate marker of left posteroseptal BTs. The tallest precordial R wave is typically recorded in the midprecordial leads (V2 to V4) in left posteroseptal BTs, and in the lateral precordial leads (V5 or V6) in right posteroseptal BTs.
Localization Using Polarity of the Retrograde P Wave Morphology
The polarity of the retrograde P waves during orthodromic AVRT is dependent on the location of the atrial insertion of the BT, and is helpful in localizing the BT. However, the P wave is usually inscribed within the ST segment and its morphology may not be easily determined.
In general, P wave morphology in leads I and V1 and in the inferior leads is most helpful ( Fig. 18.22 ). A negative P wave vector in lead I is highly suggestive of left free-wall BTs, whereas a positive vector is suggestive of right free-wall BTs. On the other hand, a negative P wave in lead V1 predicts right-sided BTs. P wave polarity in the inferior leads, positive or negative, suggests superior or inferior location of the BT, respectively.

Left free-wall BTs have positive P waves in lead V1 and negative P waves in leads I and aVL. If the retrograde P wave is negative in all three inferior leads, the BT is located at the inferoposterior mitral annulus. As the BT location moves to the lateral mitral annulus, the P wave becomes isoelectric or biphasic in one of the three inferior leads. The P wave becomes positive in all inferior leads for left anterior/anterolateral BTs. Thus, as the BT moves from posterior to anterior locations along the mitral annulus, the positive P wave is seen initially in lead III, followed by lead aVF and lead II.
For right free-wall BTs, the P wave is negative in lead V1 and positive or isoelectric in lead I. If the P wave is positive in all inferior leads, the BT is located in the anterior wall. If the P wave is negative in all inferior leads, the BT is located in the posterior wall. However, moving from the posterior to the anterior location, the positive P wave is seen initially in lead II, followed by lead aVF and lead III.
Posteroseptal BTs display positive P waves in leads V1, aVR, and aVL, and isoelectric or biphasic P waves in lead I. P waves are negative in all inferior leads (II, III, aVF). Left posterior BTs also have negative P waves in the inferior leads, but the P waves are more negative in lead II than in lead III and are more positive in lead aVR than in lead aVL. Discrimination between left and right posteroseptal BTs based on P wave morphology is limited. In contrast, anteroseptal BTs display positive P waves in inferior leads and biphasic P waves in lead V1.
Electrophysiological Testing
EP testing is used to study the features, location, and number of BTs and the tachycardias, if any, associated with them ( Box 18.3 ). Typically, three quadripolar catheters are positioned in the high RA, RV apex or septum, and HB region, and a decapolar catheter is positioned in the CS ( see Fig. 4.4 ). If a right-sided BT is suspected, a duo-decapolar (Halo) catheter along the tricuspid annulus can be helpful.
- •
Confirming the presence of an atrioventricular BT
- •
Evaluation for the presence of multiple BTs
- •
Localization of the BT(s)
- •
Evaluation of the refractoriness of the BT and its implications for life-threatening arrhythmias
- •
Induction and evaluation of tachycardias
- •
Demonstration of the BT role in the tachycardia
- •
Evaluation of other tachycardias not dependent on the presence of the BT
- •
Termination of the tachycardias
BT, Bypass tract.
Some investigators have advocated a simplified approach to ablation using only one or two catheters. Although often successful, 10% of patients with preexcitation have multiple arrhythmias, and 10% to 20% of such patients have multiple BTs that can result in a very complex procedure. Because it is difficult to know beforehand in any given patient whether the procedure will be straightforward or complex, the single-catheter approach to ablation of arrhythmias should be discouraged.
Baseline Observations During Sinus Rhythm
Ventricular preexcitation is associated with a short His bundle–ventricular (HV) or H-delta interval during NSR. The HV interval can even be negative or the His potential can be buried in the local ventricular electrogram. The QRS is a fusion between conduction over the BT and that over the AVN-HPS. The site of earliest ventricular activation is near the ventricular insertion site of the BT (i.e., near the tricuspid annulus or mitral annulus at the base of the heart). Slowing of conduction in the AVN by carotid sinus massage, AVN blockers, or rapid atrial pacing unmasks and increases the degree of preexcitation, because these maneuvers do not affect the conduction over the BT. Dual AVN pathways are present in 8% to 40% of patients.
Programmed Atrial Stimulation During Sinus Rhythm
In the presence of a manifest AV BT, atrial stimulation from any atrial site can help unmask preexcitation if it is not manifest during NSR because of fast AVN conduction. Incremental rate atrial pacing and progressively premature AES produce decremental conduction over the AVN (but not over the BT), increasing the degree of preexcitation and shortening the HV interval, until the His potential is inscribed within the QRS. The His potential remains activated anterogradely over the AVN until anterograde block in the AVN occurs; the QRS then becomes fully preexcited, and the His potential becomes retrogradely activated ( Fig. 18.23 ).

Atrial stimulation close to or at the AV BT insertion site results in maximal preexcitation and the shortest P-delta interval because of the lack of intervening atrial tissue whose refractoriness may otherwise limit the ability of atrial stimulation to activate the AV BT as early ( Fig. 18.24 ). Atrial pacing can reveal the presence of multiple BTs ( eFig. 18.4 ). Rare cases of catecholamine-dependent BTs have been reported that require isoproterenol infusion to manifest preexcitation that is absent at baseline.
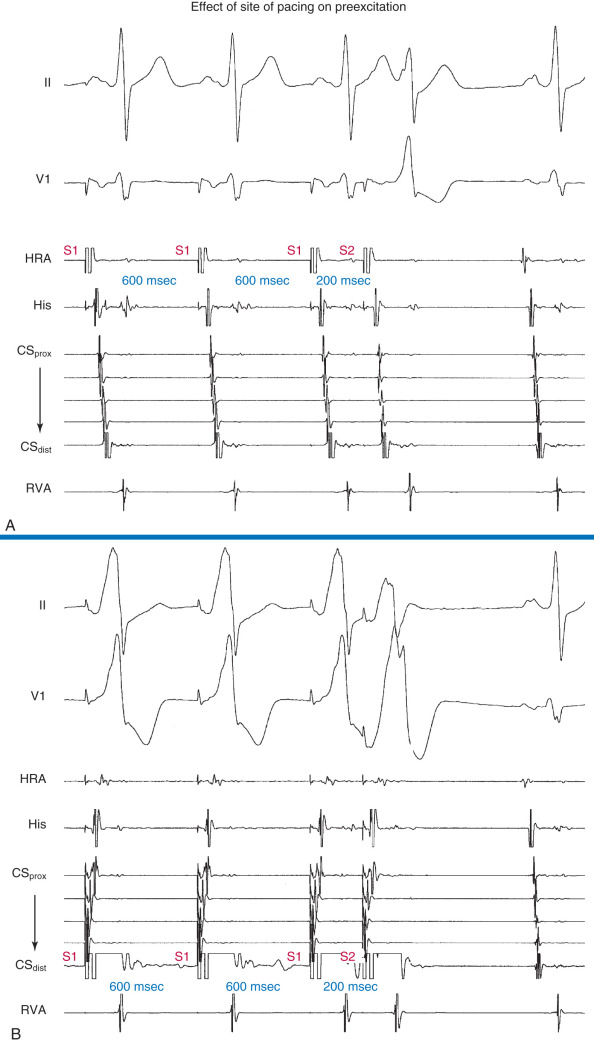

The failure of atrial stimulation to increase the amount of preexcitation can be caused by: (1) markedly enhanced AVN conduction; (2) the presence of another AV BT; (3) pacing-induced block in the AV BT because of a long ERP of the BT (longer than that of the AVN); (4) total preexcitation already present at the basal state caused by prolonged or absent AVN-HPS conduction; (5) decremental conduction in the BT; or (6) the presence of a fasciculoventricular BT rather than an AV BT.
Programmed Ventricular Stimulation During Sinus Rhythm
Retrograde ventriculoatrial conduction.
The normal AVN response to rate-incremental ventricular pacing or progressively premature single ventricular extrastimulation (VES) is a gradual delay of VA conduction (manifest as gradual prolongation of the VA and His bundle–atrial [HA] intervals) as the PCL or VES coupling interval decreases. Conversely, nondecremental VA conduction characterizes BT conduction. Nonetheless, some BTs with retrograde decremental conduction properties can also exhibit prolongation of conduction time and VA interval with ventricular pacing or VES. In addition, at short ventricular PCLs or VES coupling intervals, intramyocardial conduction delay can occur, resulting in prolongation in the VA interval; however, the local VA interval at the BT location remains unchanged. Furthermore, short ventricular PCLs or VES coupling intervals can encroach on the BT refractoriness, causing some decremental conduction, with a consequent increase in the surface VA interval and the local VA interval.
The absence of VA conduction (at long ventricular PCLs) or the presence of decremental VA conduction makes the presence of a retrogradely conducting BT unlikely, except for the rare catecholamine-dependent BTs that require isoproterenol infusion for demonstration.
Retrograde atrial activation sequence.
In the presence of a retrogradely conducting AV BT (whether manifest or concealed), VES during NSR can result in VA conduction over the BT, AVN, both, or neither ( Fig. 18.25 ). Conduction over the BT alone is the most common pattern at short PCLs or short VES coupling intervals. In this setting, the VA conduction time is fairly constant over a wide range of PCLs and VES coupling intervals (given the absence of intraventricular conduction abnormalities or additional BTs). On the other hand, retrograde conduction over both the BT and HPS-AVN is especially common when RV pacing is performed at long PCLs or long VES coupling intervals and in the presence of a left-sided BT. This occurs because it is easier to engage the RB and conduct retrogradely through the AVN than it is to reach a distant left-sided BT. In this setting, the atrial activation pattern depends on the refractoriness and conduction times over both pathways and usually exhibits a variable degree of fusion. In addition, VA conduction can proceed over the HPS-AVN alone, resulting in a normal pattern of VA conduction, or can be absent because of block in both the HPS-AVN and BT, which is especially common with short PCLs and very early VES.

Ventricular stimulation resulting in eccentric retrograde atrial activation sequence not consistent with normal conduction over the AVN is consistent with VA conduction over an AV BT (see Fig. 18.25 ). Ventricular pacing can also reveal the presence of multiple BTs ( Fig. 18.26 ). However, a concentric retrograde atrial activation sequence does not exclude the presence of a septal or paraseptal BT or a free-wall BT when VA conduction is mediated by the AVN. In addition, AVN slow pathway conduction can be associated with an eccentric atrial activation sequence in the CS. Accurate analysis of an atrial activation sequence frequently requires the use of multielectrode catheters around the tricuspid annulus and deep in the CS.

VES during HB refractoriness.
A VES delivered when the HB is refractory (i.e., when the His potential is already manifest or within 35 to 55 milliseconds before the time of the expected His potential) that results in atrial activation is diagnostic of the presence of a retrogradely conducting BT. Because the HPS-AVN is already refractory and cannot mediate VA conduction, retrograde atrial activation from ventricular stimulation has to be mediated by a BT.
Furthermore, an early coupled VES that results in an atrial activation that either precedes HB activation ( Fig. 18.27 ) or is associated with an apparent HA interval shorter than that during drive complexes indicates “atrial preexcitation” via an AV BT.

Importantly, if a VES delivered when the HB is refractory does not result in atrial activation, this does not necessarily exclude the presence of a retrogradely conducting AV BT, because such a VES can be associated with retrograde block in the BT itself (see Fig. 18.25 ). In addition, the lack of such a response does not exclude the presence of unidirectional (anterograde-only) AV BTs.
Retrograde RBBB during VES.
During the delivery of progressively premature single VESs, an abrupt increase in the VA conduction interval is often observed. This can be due to a variety of reasons including: (1) retrograde block in the AVN fast pathway and subsequent VA conduction over the slow pathway; (2) retrograde block in the RB and subsequent retrograde conduction over the left bundle branch (LB); or (3) retrograde block in the BT and subsequent VA conduction only over the AVN.
Retrograde RBBB occurs frequently during VES testing, and can be diagnosed by observing the retrograde His potential during the drive train and its abrupt delay following the VES. Often, however, it is difficult to visualize the retrograde His potential during the pacing train; nevertheless, the sudden appearance of an easily distinguished retrograde His potential, separate from the ventricular electrogram following the VES, can be sufficient to recognize retrograde RBBB.
Prolongation of the ventriculo-His (VH) interval is observed on development of retrograde RBBB because conduction must traverse the interventricular septum (which requires approximately 60 to 70 milliseconds in normal hearts), enter retrograde via the LB, and ascend to reach the HB. Although an increase in the VH interval necessarily occurs with retrograde RBBB, whether a similar increase occurs in the VA interval depends on the nature of VA conduction (over the AVN vs. BT).
Measurement of the effect of the development of retrograde RBBB during VES on the retrograde VH and VA intervals can help distinguish between retrograde AVN and BT conduction. In the absence of a BT, the AVN can be activated in a retrograde fashion only after retrograde activation of the HB; as a consequence, VA activation will necessarily be delayed with retrograde RBBB, and the increase in the VA interval will be similar to the increase in the VH interval. On the other hand, when retrograde conduction is via a BT, there will be no expected increase in the VA interval when retrograde RBBB is induced. Thus the increase in the VA interval is minimal and always less than the increase in the VH interval.
Differential-site RV pacing.
The response of the VA interval (i.e., the stimulus-to-atrial [SA] interval) and atrial activation sequence to differential-site RV pacing (i.e., RV basal versus RV apical pacing) can help demonstrate or exclude the presence of a retrogradely conducting septal AV BT ( Fig. 18.28 ).





