Type IV Thoracoabdominal Aneurysm Repair
Mark Conrad
Indications
Thoracoabdominal aneurysms (TAA) develop through a complex process involving a combination of genetic and environmental factors. Approximately 80% are degenerative, 15% to 20% are secondary to chronic dissection and the remaining have other etiologies such as infection or aortitis. TAA are classified according to the scheme originally devised by Crawford (Fig. 16.1). This classification is especially useful in patients requiring operative repair since it has direct implications for both the technical conduct of the operation, and the incidence of operative complications; in particular, ischemic spinal cord injury. This chapter will focus on Type IV TAA which have an upper limit of the crus of the diaphragm (or 13th intercostal space) and can extend to the iliac bifurcation.
The critical concern with TAA is rupture, which is associated with a mortality that approaches 90% and accounts for 30% of all deaths in patients with TAA. The strongest predictor of rupture is aneurysm size. Natural history studies have shown that the risk of rupture is negligible in TAA less than 5 cm, equivalent to the risks associated with open surgery in the 5 to 6 cm range, and increases substantially when the diameter exceeds 6 cm and/or the rate of interval growth is ≥10 mm per year. These observations have led to the acceptance of 6 cm as a generally appropriate size threshold for recommendation of surgical intervention for Type IV TAA. Other risk factors for rupture include increasing patient age, female gender, pain (including atypical pain), a history of chronic obstructive pulmonary disease (COPD), symptoms attributable to the aneurysm, and dissection developing within a degenerative aneurysm. Population-based studies have indicated an overall 5-year rupture risk of 20%, with 80% of ruptures occurring in women.
Contraindications
Patient selection for open repair of Type IV TAA is predicated upon weighing the likelihood of aneurysm rupture against the risk of surgery as conferred by the patient’s medical history and active comorbidities. An accurate assessment of associated comorbid conditions is mandatory to guide appropriate decision making with respect to recommending open surgical
repair. Patients with active cardiac disease, capacity-limiting COPD, and renal failure are at highest risk for postoperative complications and death after open TAA repair. These are patients in whom a nonstandard open (hybrid) repair or no intervention may be warranted.
repair. Patients with active cardiac disease, capacity-limiting COPD, and renal failure are at highest risk for postoperative complications and death after open TAA repair. These are patients in whom a nonstandard open (hybrid) repair or no intervention may be warranted.
Medical Evaluation
All patients should be evaluated with dipyridamole thallium scanning or the equivalent thereof to assess perioperative myocardial ischemic potential. In addition, patients with a history of symptoms suggestive of heart failure should have an assessment of left ventricular function. While patients with significant impairments of pulmonary reserve can usually be detected on a historical basis alone, we routinely obtain preoperative pulmonary function studies. Advanced age is an important component only in as much as it is accompanied by overall fragility and impaired functional status (both of which have been shown to predict mortality).
Imaging
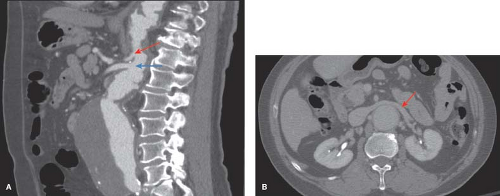
Associated vascular diseases and comorbid conditions are commonplace in patients presenting for treatment of thoracic aneurysms. Synchronous aneurysms typically involving the ascending aorta or arch have been observed in some 10% of patients being evaluated for descending thoracic aneurysm repair. Prior operation for aortic aneurysm disease is seen in one-third of patients and as noted above, the most common of these is a previous infrarenal aneurysm repair. Additionally, up to one-quarter of patients with thoracic aortic disease have concomitant abdominal aortic aneurysm (AAA), therefore all patients should undergo baseline imaging of the abdominal and pelvic aorta at the time of initial diagnosis. In contemporary practice, a dynamic, fine-cut, contrast-enhanced CT scan with or without helical reconstruction is the preferred imaging modality and provides: (1) Accurate assessment of aneurysm size and extent, (2) a baseline study to which future images may be compared, and (3) important anatomic information such as the anatomy and topography of the visceral segment, relationship of the left renal vein to the aorta (anterior or posterior) and appropriate areas for clamping (Fig. 16.2).
Accurate assessment of the size of Type IV TAA is contingent upon measurement in the appropriate perpendicular plane. Three-dimensional reconstruction of the thoracic aorta with determination of the centerline of flow ensures that any cross-sectional measurement will be perpendicular to the aortic axis. The quality of current CT scanners with three-dimensional
reconstruction is exceptional and in our practice, this has become the image modality of choice for the evaluation and treatment of thoracic aortic aneurysms (Fig. 16.3).
reconstruction is exceptional and in our practice, this has become the image modality of choice for the evaluation and treatment of thoracic aortic aneurysms (Fig. 16.3).
Principles of Treatment
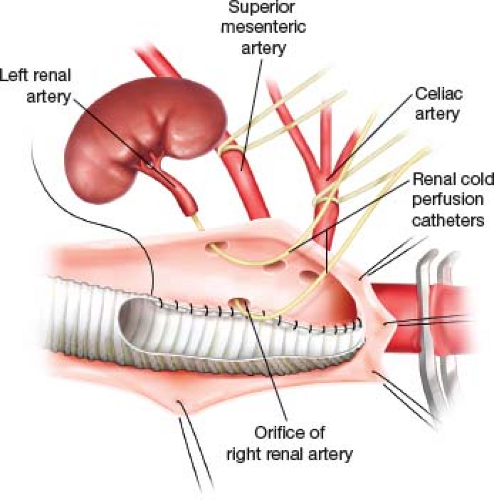
Our general approach to the repair of Type IV TAA is the clamp-and-sew technique. Hypothermic renal protection is usually employed. A bolus (250 cc) of renal preservation
fluid (4°C lactated Ringers with 25 g of mannitol per liter and 1 g methyl prednisolone per liter) is infused directly into the ostia of each renal artery after the aorta is opened. This is followed by a continuous drip of the same delivered through 6-Fr perfusion balloon-tipped catheters. The initial bolus results in a decline of renal parenchymal temperature to 15°C and the subsequent continuous infusion maintains the renal core temperature around 25°C as measured by direct temperature probes in the renal cortex (Fig. 16.4).
fluid (4°C lactated Ringers with 25 g of mannitol per liter and 1 g methyl prednisolone per liter) is infused directly into the ostia of each renal artery after the aorta is opened. This is followed by a continuous drip of the same delivered through 6-Fr perfusion balloon-tipped catheters. The initial bolus results in a decline of renal parenchymal temperature to 15°C and the subsequent continuous infusion maintains the renal core temperature around 25°C as measured by direct temperature probes in the renal cortex (Fig. 16.4).
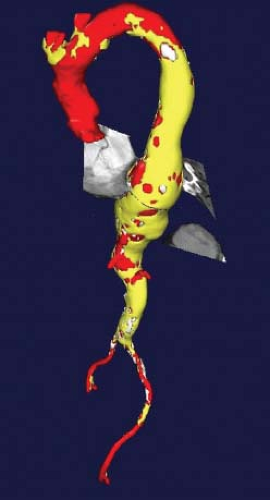 Figure 16.3 Three-dimensional reconstruction of the thoracoabdominal aorta with an axial window in the centerline plane allows for the most accurate measurement of aneurysm diameter. |
Positioning
Irrespective of individual preferences concerning the technical components of the operation, the key to operative success remains the provision of broad, continuous exposure of the entire left posterolateral aspect of the thoracoabdominal aorta. The patient is positioned on the table in the right lateral decubitus position. The location and extent of the thoracic portion of the incision is determined by the proximal extent of the aneurysm. A thoracoabdominal incision at the 8th interspace will usually provide adequate exposure for a Type IV aneurysm, and a double lumen tube for deflation of the left lung is generally not necessary in these cases (Fig. 16.5




Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree