Fig. 2.1
A model showing type II cell progenitor functions following alveoli injury. (a) Schematic representation of alveoli. (b) Section of mouse lung alveoli region stained by type II cell marker Sp-C (red) and type I cell marker T1α (green). Scale bar = 50 μm. (c) Upon alveolar epithelial injury, some type II cells are activated, undergo proliferation and transition into type I cells to restore alveolar barrier integrity
Alveolar type II cells have multiple functions. One of the most important functions of these cells is to synthesize and secrete surfactant (Mason and Shannon 1997; Mason 2006). Surfactants are synthesized in type II cell-specific organelles called “lamellar bodies.” Each type II cell contains approximately 150 lamellar bodies. This structure has a mean diameter of 1 μm, contains multiple phospholipid bilayers, and gives type II cells a unique morphology (Mason and Shannon 1997; Mason 2006; Mason and Crystal 1998). In this organelle, the phospholipids are packed with the surfactant proteins Sp-A, Sp-B, Sp-C, and Sp-D, which constitute surfactant (Andreeva et al. 2007; Mason and Shannon 1997). When secreted into the alveolar space, surfactant maintains alveolar surface tension and prevents lung collapse (Mason and Shannon 1997; Mason and Crystal 1998). While Sp-A, Sp-B, and Sp-D are also produced in some bronchiolar cells (Mason and Shannon 1997; Walker et al. 1986; Fehrenbach 2001), Sp-C is produced only in type II cells and is considered a type II cell marker (Kalina et al. 1992; Mason and Shannon 1997; Fehrenbach 2001).
Other than producing surfactant, type II cells also play roles in the transepithelial transport of ions and fluids (Mason and Shannon 1997; Mason 2006), modulation of lung inflammatory responses (Mason 2006), and maintenance of epithelial homeostasis and integrity by acting as progenitor cells through proliferation and differentiating into alveolar type I cells (Mason and Shannon 1997; Mason 2006; Stripp 2008; Rock and Hogan 2011). The function of type II cells as alveoli epithelial progenitor cells during injury repair is discussed in this chapter.
Type II cell hyper-proliferation is an important feature in the resolution and repair phase following lung injury (Matthay et al. 2012; Ware and Matthay 2000; Shimabukuro et al. 2003). Evidence indicates that type II cells function as progenitor cells for the re-epithelialization of injured alveoli by converting into type I cells (Evans et al. 1973, 1975) (Fig. 2.1c). In the 1970s, Evans et al. performed classical experiments using NO2 to damage the alveolar surface and showed that 3H-TdR was first incorporated into proliferating type II cells and later labeled type I cells (Evans et al. 1973, 1975). In addition, it has been shown that isolated type II cells can differentiate into type I cells in culture (Dobbs 1990). However, knowledge of the detailed cellular and molecular mechanisms of the progenitor cell properties of type II cells has only recently started to emerge.
2.2 Type II Cells Exhibit Progenitor Cell Phenotypes In Vitro
No reported cell line exhibits all the major properties of type II cells. However, techniques have been developed to isolate type II cells from human, rat, and mouse lungs (Kikkawa and Yoneda 1974; Dobbs 1990; Corti and Brody 1996). Basically, lung samples are separated into single cell suspensions using enzymes, such as dispase, and the type II cells are enriched using differential sedimentation. The type II cells can be further purified using antibodies to surface antigens of different cell types (Dobbs 1990; Corti and Brody 1996) (Fig. 2.2a).
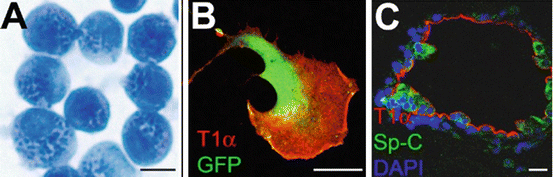

Fig. 2.2
Type II cells are able to differentiate into type I cells in culture. (a) Freshly isolated mouse type II cells stained with modified Papanicolaou method (Dobbs 1990). Dark blue dots are the lamellar bodies (Dobbs 1990). (b) After culturing on plastic surface, some type II cells underwent transition into type I-like cell and expressed type I cell marker T1α. This cell also expressed GFP in cytoplasm. (c) When co-cultured with lung fibroblasts in 3D matrigel, some type II cells formed alveoli-like structures that contain Sp-C expressing type II cells and T1α expressing type I cells. Scale bar = 10 μm
Isolated type II cells can be cultured on mixture of collagen, laminin, or fibronectin substrata on which they maintain their type II cell phenotypes (such as forming lamellar bodies and secreting surfactant) for approximately 1 week; however, the cells have a tendency to differentiate into flat type I-like cells (Dobbs 1990; Rice et al. 2002; Gobran and Rooney 2004; Rannels et al. 1987; Paine and Simon 1996). If isolated type II cells are cultured on plastic in the presence of 5–10 % fetal bovine serum and without exogenous extracellular matrix, they undergo a morphological change, lose their lamellar bodies, and differentiate into flat type I-like cells within 2–7 days (Dobbs 1990). Some of these type II cell-derived flat cells express type I cell markers such as aquaporin 5 and T1α (Williams 2003) (Fig. 2.2b). Thus, in vitro studies have suggested that type II cells may be precursors of type I cells.
Recently, a new 3D culturing technique of culturing type II cells has been developed (Barkauskas et al. 2013; McQualter et al. 2010; Chen et al. 2012a). When isolated type II cells are cultured in Matrigel (a mixture of extracellular matrix components secreted by Engelbreth-Holm-Swarm (EHS) mouse sarcoma cells) (Kleinman and Martin 2005), they form cysts consisting of polarized monolayers of type II cells, and the cells secrete surfactant into the lumen (Dodelet and Pasquale 2000). However, when type II cells are co-cultured with lung mesenchymal cells in Matrigel (Barkauskas et al. 2013; McQualter et al. 2010; Chen et al. 2012a), they form 3D alveoli-like structures with flat type I-like cells lining the lumen and cuboidal type II-like cells facing the matrix (Fig. 2.2c). By mixing cells from different lineage-labeled lines, it appears that each of the alveolar-like spheres can be derived from a single cell (Barkauskas et al. 2013). These results show that at least some subgroups of type II cells have stem cell characteristics that include the ability to expand clonally and differentiate into multiple lineages.
From the above studies, the behavior of type II cells varies depending on the culture conditions. Developing new culture techniques will lead to novel discoveries of the progenitor cell aspects of type II cells. Some semi-in vivo culture methods, such as growing cells in subcutaneous Matrigel plugs or in a renal capsule (Chapman et al. 2011; Lee et al. 2014), may provide additional insight into type II cell progenitor functions.
2.3 Type II Cells Act as Progenitor Cells During Lung Repair in Animal Models
To study the progenitor cell behavior of type II cells during alveolar repair in vivo, it is important to have proper animal models to mimic various alveolar injuries; these studies also provide a bridge between patients and laboratory research. Because of their size and the availability of genetic approaches, mouse and rat models are widely used. Typically, three types of agents are used to target the alveolar epithelium in experimental animals: (1) the inhalation of gasses, such as toxic NO2 (Evans et al. 1973, 1975); because high concentrations of O2 can cause alveolar damage in rodents, hyperoxia is a frequently used alveolar injury model (Pogach et al. 2007); (2) the administration of chemicals or antibiotics, such as acid (Matute-Bello et al. 2008) or bleomycin (Flozak et al. 2010); and (3) the introduction of pathogens such as the intratracheal injection of Pseudomonas aeruginosa bacteria (Sadikot et al. 2005; Liu et al. 2011) or the H1N1 influenza virus (Kumar et al. 2011) (see Table 2.1).
Table 2.1
Selected animal models of alveolar epithelial injury
Model | Mechanism of injury | Lung features (emphasizing type II cells) | References |
|---|---|---|---|
Inhalation of toxic gas NO2 | Generation of nitrating oxidants; subsequent inflammation | Acute injury; type II cell proliferation and transition into type I cells | |
Hyperoxia | Generation of reactive oxygen species; inflammation; cell necrosis or apoptosis; degradation of extracellular matrix | Acute lung injury; neutrophilic infiltration followed by type II cell proliferation; fibroblast proliferation and scarring | |
Acid aspiration | Cell death; disruption of the alveolar/capillary barrier; neutrophilic infiltration | Acute lung injury and inflammation; type II cell proliferation, heal with fibrosis | |
Bleomycin | Formation of complex with metal and oxygen; production of oxygen radicals; inflammation | Inflammation and acute alveoli injury; type II cell proliferation and conversion to type I cells; later lead to reversible fibrosis | |
Intrapulmonary bacteria, e.g., intratracheal inject live P. aeruginosa | Bacteria and the toxin damage cell; neutrophil infiltration; inflammatory response | Acute lung injury and inflammatory response;increased alveoli barrier permeability; type II cell proliferation and differentiation into type I cells; lung repaired after 7 days | |
Viral infection, e.g., H1N1 | Destruction of alveoli cells; excessive inflammatory responses | Trp63+ cells proliferate and produce pod-like structures in alveoli | |
Diptheria toxin | Toxin kill cells; no apparent inflammation | Remaining type II cells proliferate and transit into type I cells | Barkauskas et al. (2013) |
Unilateral pneumonectomy | Mechanical injury | Compensatory growth of existing lobes; type II cell proliferation | Nolen-Walston et al. (2008) |
The mechanisms that cause alveolar damage vary in different injury models. NO2 causes cell death by nitrating oxidants and the subsequent inflammatory responses (Persinger et al. 2001). In the hyperoxia model, high oxygen concentrations (usually 80–100 %) release reactive oxygen species or free radicals derived from O2, causing necrosis or apoptosis of alveolar epithelia cells (Pagano and Barazzone-Argiroffo 2003). Hyperoxia also leads to the activation of NFκB signaling and inflammatory responses that further enhance alveolar damage (Matute-Bello et al. 2008). One limitation of the hyperoxia model is that, in humans with normal lungs, exposure to 100 % oxygen does not cause clinical or pathological lung injury (Matute-Bello et al. 2008). The acid aspiration model is usually accomplished by the instillation of HCl into the trachea. The acid causes alveolar cell death, increased alveolar barrier permeability and an acute inflammatory response (Matute-Bello et al. 2008). Bleomycin is an antineoplastic antibiotic (Matute-Bello et al. 2008; Wansleeben et al. 2013) that, when administered into the lung, forms a complex with oxygen and metals, such as Fe, leading to the production of oxygen radicals that cause alveolar cell death (Matute-Bello et al. 2008; Wansleeben et al. 2013). At the same time, the bleomycin-treated lung undergoes an inflammatory response that increases damage (Wansleeben et al. 2013; Matute-Bello et al. 2008). The intratracheal instillation of bacteria causes pneumonia. Some bacteria such as Pseudomonas aeruginosa produce toxins that penetrate the cell membrane, causing cell death (Sadikot et al. 2005). The H1N1 influenza virus causes cell death and an inflammatory response (Wansleeben et al. 2013; Hendrickson and Matthay 2013; Kumar et al. 2011) (Table 2.1).
Excessive inflammation is a factor common to most of these models and plays an important role in causing cellular damage (Matute-Bello et al. 2011). The only exception listed in Table 2.1 is a model of diphtheria toxin-induced lung injury (Barkauskas et al. 2013). Using a knock-in mouse in which the expression of diphtheria toxin in type II cells is induced following tamoxifen injection, there does not appear to be a clear inflammatory response (Barkauskas et al. 2013) (Table 2.1).
Some types of injuries are reversible; for example, Pseudomonas aeruginosa-induced lung injury is usually repaired in approximately 7–10 days (Liu et al. 2011). By contrast, some injuries lead to chronic disease; for example, bleomycin injection can induce acute lung injury in the early phase; later, the lung frequently develops fibrosis (Flozak et al. 2010; Chapman et al. 2011; Zhao et al. 2002). Recent findings indicate that the lung alveoli may utilize different repair mechanisms in response to different types of damage. For example, a group of putative progenitor cells called BASC (for bronchioalveolar stem cells) appear to migrate and proliferate in response to bleomycin-induced injury (Barkauskas et al. 2013), but they do not respond to hyperoxia (Rawlins et al. 2009). This result is not surprising because the pathological mechanism as well as the extent of injury varies in different models. Most agents cause damage in multiple cell types in the alveoli, some injuries may be localized to certain foci, and others affect a more extended region. One might hypothesize that certain local facultative progenitor cells may be sufficient to repair a local mild injury, while putative stem cells reside in certain niches in the alveoli or airway may be mobilized in response to a more severe injury (Vaughan and Chapman 2013). Therefore, it is important to compare several injury models to study the mechanisms of alveolar repair.
Type II cells have been shown to be involved in repair in different injury models (Table 2.1). In response to NO2, type II cells proliferate, as revealed by 3H labeling (Evans et al. 1973, 1975). In addition, in pulse labeling experiments, the 3H label appeared first in type II cells and later in type I cells, suggesting that type II cells give rise to type I cells (Evans et al. 1973, 1975). Following hyperoxia or acid aspiration, type II cells engage in hyper-proliferation (Lee et al. 2006; Pogach et al. 2007; Desai et al. 2014). Following bleomycin-induced injury, type II cells proliferate, and lineage tracing experiments using this injury model showed that type II cells also differentiate into type I cells (Barkauskas et al. 2013; Rock et al. 2011). In a Pseudomonas aeruginosa-induced injury model (Liu et al. 2011), type II cells undergo proliferation in the early phase and differentiate into type I cells in the later repair phase (Liu et al. 2011). Finally, in the unilateral pneumonectomy (PNX) model, type II cell proliferation also plays an important role in the compensatory growth of the remaining lung (Nolen-Walston et al. 2008). Therefore, the involvement of type II cells is a common repair mechanism following alveolar injury. However, the exact molecular mechanisms and the subgroups of type II cells that are involved in the repair of different injuries may differ.
2.4 Lineage Analysis of Type II Cells Involved in Alveoli Repair
Even though the historical experiments by Evans et al. discussed above (Evans et al. 1973, 1975) strongly suggested that type II alveolar cells behave as progenitor cells by proliferating and differentiating into type I cells following injury, it is only with the recent development of new genetic approaches that direct evidence has shown that type II cells give rise to type I cells during alveolar homeostasis and repair (Rock et al. 2011; Barkauskas et al. 2013; Desai et al. 2014).
By adopting the yeast Cre DNA recombination enzyme and the cis-element loxP site (Soriano 1999), new genetic lineage tracing techniques have been developed in mouse models by coupling three elements together: an inducible Cre recombinase-expressing system; a type II cell-specific promoter; and a reporter allele tagged with a fluorescent protein (Kretzschmar and Watt 2012). Figure 2.3 shows an example of this system. With the administration of certain chemicals, in this case, tamoxifen, into mice, Cre recombinase is expressed in Sp-C-expressing type II cells, and Cre activates the expression of a fluorescent protein, such as GFP, which will permanently label the type II cells in adult mice; the fate of these labeled cells can be traced any time thereafter (Rock et al. 2011; Desai et al. 2014). Therefore, even if these type II cells later differentiate into another cell type, the cells can still be visualized by the fluorescent signals.
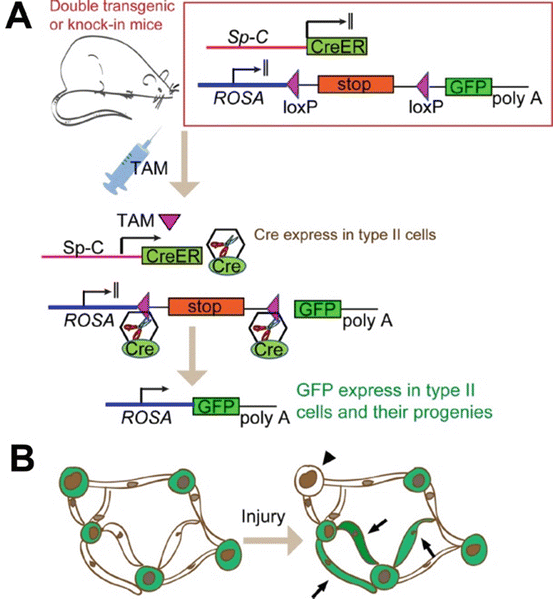

Fig. 2.3
A typical lineage tracing study shows progenitor function of type II cells. (a) A double transgenic (or knock-in) mouse harbors (1) an inducible Cre-expressing system, in this case a Cre-ER in which the expression of Cre recombinase is dependent on tamoxifen (TAM). The Cre expression is also under the control of a type II cell-specific promoter Sp-C; (2) a reporter tagged with a fluorescent protein (in this case GFP). But GFP is normally not expressed because a stop sequence flanked by two loxP sites reside upstream of it. With the administration of TAM into this mouse, Cre is expressed in Sp-C-expressing type II cells, and Cre deletes the stop sequence to activate the GFP expression in type II cells through the constitutive ROSA promoter, and then GFP will permanently label the type II cells as well as their progenies. (b) Schematic representation of a typical result of the lineage tracing studies. Mice were injected with TAM before injury, so that the type II cells were permanently labeled with GFP while type I cells were unlabeled. Following alveoli injury, some type II cells underwent transition into type I cells; therefore, some GFP-labeled type I cells appeared (arrow). In addition, some type II cells without GFP labeling appeared in the lung (arrowhead), suggesting that some Sp-C− cells gave rise to type II cells
Using this lineage tracing mouse line and low doses of tamoxifen, Barkauskas et al. showed that only a small number of type II cells were labeled with the fluorescent proteins. When traced after 12–30 weeks, it was found that these labeled cells formed small clusters, indicating that type II cells undergo a slow self-renewal (Barkauskas et al. 2013). The same studies also showed that the labeled type II cells slowly differentiated into type I cells during steady-state tissue maintenance. Immediately after the mice were injected with tamoxifen, only type II cells were labeled by fluorescent proteins. However, after a 24-week chase, some flat cells expressing type I cell markers were also labeled with the same fluorescent signals, indicating that the lineage-labeled type II cells had converted into type I cells. These results are consistent with another lineage tracing study using a different mouse line (Desai et al. 2014) and support the conclusions from earlier cell tracing studies using 3H-labeled thymidine showing that the steady state turnover time of type II cells is on the order of 4–6 weeks (Spencer and Shorter 1962; Fehrenbach 2001).
From these studies, type II cells have been shown to be relatively quiescent in the absence of injury. However, in response to alveolar injury, type II cells are activated and enter the repairing program (Mason and Shannon 1997; Barkauskas et al. 2013; Desai et al. 2014). Using the above lineage tracing mouse lines, it is shown that after injury, the conversion of type II cells into type I cells was greatly accelerated (Barkauskas et al. 2013).
Furthermore, in another study that traced the type II cells following Pseudomonas aeruginosa-induced injury, it is found that some lineage-labeled type II cells lost the type II cell marker Sp-C at 5–7 days post-injury, and these cells did not yet express or only expressed faint level of type I cell markers. These may represent the intermediate cells undergoing type II to I transition (Liu et al. 2011).
Another question related to the progenitor function of type II cells in repair is how injured type II cells are replenished. Because type II cells undergo hyper-proliferation following injury (Mason and Shannon 1997), one might hypothesize that type II cells are able to repair themselves solely by self-renewal. However, recent lineage tracing studies have shown that type II cells may not be the only source for self-replenishment (Barkauskas et al. 2013; Chapman et al. 2011). It has been shown using lineage tracing mouse lines (Fig. 2.2), that, following bleomycin-induced injury, the percentage of lineage-labeled type II cells versus the total number of type II cells decreased in the injured areas. These results indicate that some non-lineage-labeled cells, i.e., cells that did not express Sp-C before the injury, gave rise to type II cells during repair (Barkauskas et al. 2013; Chapman et al. 2011).
2.5 Subgroups of Type II Cells Display Progenitor Features
Type II cells have long been considered a homogeneous population. However, recent studies have indicated that type II cells are heterogeneous and consist of subpopulations. For example, studies using transgenic mice that express GFP under the control of the Sp-C promoter have revealed that certain type II cells express higher levels of GFP than others (Lee et al. 2013; Roper et al. 2003). It would, therefore, be informative to separate type II cells into subpopulations because some of these subgroups may be particularly important in the repair of injured alveoli.
It has been found that approximately 5–10 % of type II cells express low levels of CC10. CC10, also known as CCSP or Scgbb1a1, is a secretory protein expressed at high levels in Club cells located in the airways. A number of Sp-C+CC10+ type II cells are located in the alveolar region; the function of these cells is unknown. A rare population of SP-C+CC10+ cells located at terminal bronchioles is known as BASC. BASCs show certain stem cell properties in vitro including forming colonies when co-cultured with mouse embryonic fibroblasts (MEFs) (Kim et al. 2005a, b) as well as multi-lineage differentiation when cultured in Matrigel (Kim et al. 2005a, b; Lee et al. 2014).
Some subpopulations of type II cells appear following injury. Studies by Reddy et al. showed that type II cells isolated from hyperoxia-treated rats can be separated into two populations after a brief culture; some cells expressed lower levels of E-cadherin than others (Driscoll et al. 2000; Reddy et al. 2004). Furthermore, the E-cadherinhigh population appeared to be relatively quiescent, while the E-cadherinlow population was more proliferative and expressed higher levels of telomerase activity (Driscoll et al. 2000). Therefore, certain type II cells appear to show more progenitor cell properties than their counterparts.
A subgroup of type II cells expressing the progenitor cell marker Sca-1 appear in the early repair phase following Pseudomonas aeruginosa-induced injury (Liu et al. 2011). These cells are mostly derived from Sp-C-expressing type II cells and can account for more than 40 % of the total type II cells following injury (Liu et al. 2011). This Sca-1+ subpopulation of type II cells exhibits a higher proliferation rate and also has a higher potential to convert into type I cells compared with the Sca-1− counterpart (Liu et al. 2011, 2014). These cells express higher levels of the transcription factor FoxM1 than the Sca-1− type II cells, and FoxM1 is required for the alveolar repair process (Liu et al. 2011). Thus, the Sca-1+ subgroup of type II cells appear to be responsible for the repair.
The results from the above studies indicate that certain subpopulations of type II cells manifesting progenitor cell features appear at the repair phase following injury. However, it is unclear whether these putative regenerative type II cells appearing post-injury are derived from the expansion of existing stem cell pools located in an undefined niche or whether they are derived from quiescent, terminally differentiated type II cells. It would also be interesting to identify which signals or factors induce the formation of progenitor-like type II cell subgroups following injury.
Another point is that, in most studies, Sp-C is considered a type II cell-specific marker. In other words, type II cells are defined as Sp-C+ cells. However, it has been observed that some type II cells express lower levels of Sp-C than others (Chapman et al. 2011; Liu et al. 2011; Lee et al. 2013). In addition, it has been reported that a small number of epithelial cells located in alveoli do not show type I cell phenotypes and do not express detectable levels of Sp-C (Chapman et al. 2011). It remains to be determined whether these cells can be regarded as a subtype of type II cells or type II-like cells. Among the Sp-C− non-type I alveolar epithelial cells is a group of cells expressing the α6β4 integrin (Chapman et al. 2011); α6β4 integrin double-positive cells have progenitor cell features and may participate in repair following bleomycin-induced injury (Chapman et al. 2011).
2.6 Cellular and Molecular Mechanisms Regulating Type II Cell Progenitor Properties
The cellular and molecular mechanisms underlying how type II cells engage in the repair of a damaged alveolar barrier is unclear. It is known that type II cells can convert into type I cells; however, the detailed cellular processes involved in this transition are unknown. One possibility is that certain relatively undifferentiated subgroups of type II cells located at unknown locations differentiate into type I cells. Another possibility is that differentiated, mature type II cells undergo trans-differentiation and give rise to type I cells. It is also possible that mature type II cells first undergo dedifferentiation into intermediate precursors and then differentiate into type I cells. Electron microscopy studies suggest that there are intermediate cell types appearing in NO2-injured lung that show morphological characters of both type II and type I cells (Evans et al. 1975). It has also been reported that alveolar cells co-expressing type II and type I cell surface markers appear following Staphylococcus aureus-induced lung injury (Clegg et al. 2005). The existence of intermediate cells supports the transition of type II to type I cells.
A possible mechanism to initiate type II cell activation into the repair process may be signals related to the injury. Recent studies have suggested that the inflammatory milieu that forms following most types of alveolar injury can create alveolar regenerative signals (Pociask et al. 2013; Buckley et al. 2011). In injuries induced by hyperoxia, the generation of oxidants may also signal the initiation of the repair process (Pogach et al. 2007). Type II cells, in response to unknown signals related to specific injuries, can start proliferation and a type I cell transition, and they may also migrate to the injured region and engage in repair.
Little is known regarding the molecules that regulate the type II cell activation that leads to alveolar repair. One would expect that the transcriptional programs involved in embryonic lung development, such as those under the control of the FGF and Wnt pathways (Cardoso 2001; Morrisey and Hogan 2010; Warburton et al. 2008), may be activated post-injury and may contribute to repair. However, even though FGF and Wnt signaling appear to be involved in alveolar repair (Tanjore et al. 2013; Flozak et al. 2010; Ghosh et al. 2013), several transcription factors (Id2, Erm, Gata6, and Elf5) that are involved in alveolar development have not been shown to have elevated expression in type II cells following Pseudomonas aeruginosa-induced injury (Liu et al. 2011; Liu and Hogan 2002). Therefore, even if there may be some correlations, the repair process does not completely recapitulate the developmental process in embryogenesis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


